Moderna has sought the US FDA's emergency authorization for its mRNA Covid-19 vaccine for use in adolescents. Last month the FDA cleared Pfizer's request for use of its Covid-19 vaccine in adolescents.
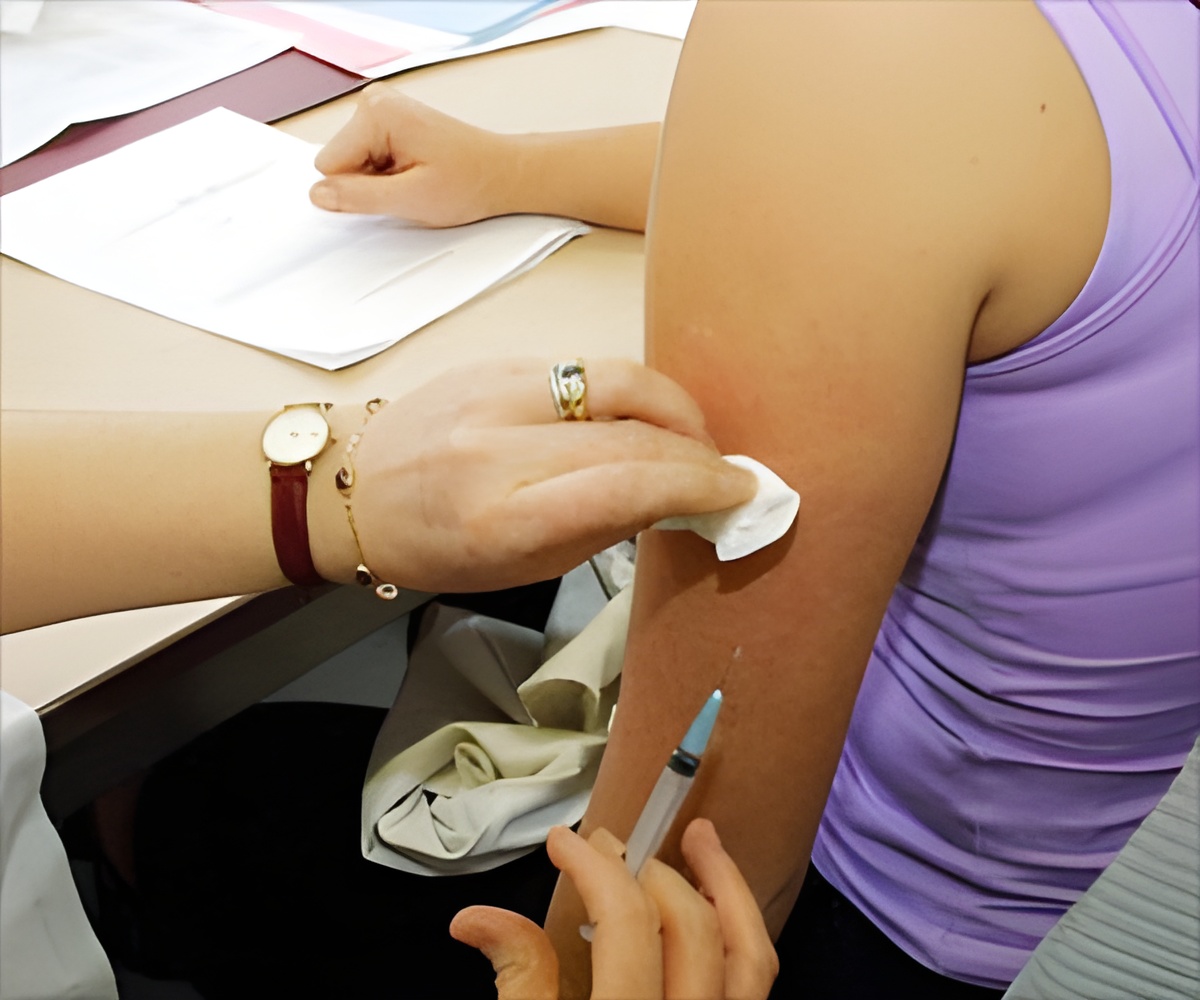
‘Last month the FDA cleared Pfizer's request for use of its Covid-19 vaccine in adolescents and about 7 million children under 18 have received at least one dose of the vaccine so far.’





"We are encouraged that the Moderna Covid-19 vaccine was highly effective at preventing Covid-19 and SARS-CoV-2 infection in adolescents," Bancel added. The Moderna Covid-19 Vaccine was approved for emergency use in the US in December. Its application to the US FDA for young teenagers is based on a study, which enrolled 3,732 children ages 12 to 17 years. Of these, 2,500 received two doses of the vaccine and the remaining a saltwater placebo.
The results reported last month showed Moderna's Covid-19 vaccine was effective in adolescents. There were no cases of symptomatic Covid-19 among vaccine recipients, Moderna said, suggesting 100 per cent vaccine efficacy in adolescents, though overall very few among the 3,700 children in the study got sick.
The company has already filed for authorization with Health Canada and the European Medicines Agency, and plans to seek approval in other countries.
Last week, the company applied to the US FDA for full approval for its mRNA Covid-19 vaccine for use in people 18 and older.
Advertisement
In addition, the company is also planning a third booster shot. According to data from its Phase 2 study, antibodies produced by a single 50 mg dose of its booster shot was effective against the original form of the virus, as well as against two variants of concern Beta (B1351) and Gamma (P1) first identified in South Africa and Brazil.
Advertisement
Source-IANS