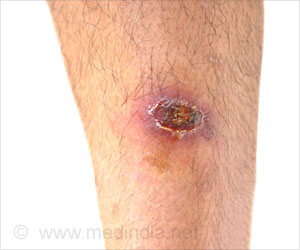
Carried out in Benin, Ghana, India, Mexico, Nigeria, Rwanda and South Africa, the FALCON trial was done to identify proper guidance in treating wound infections.
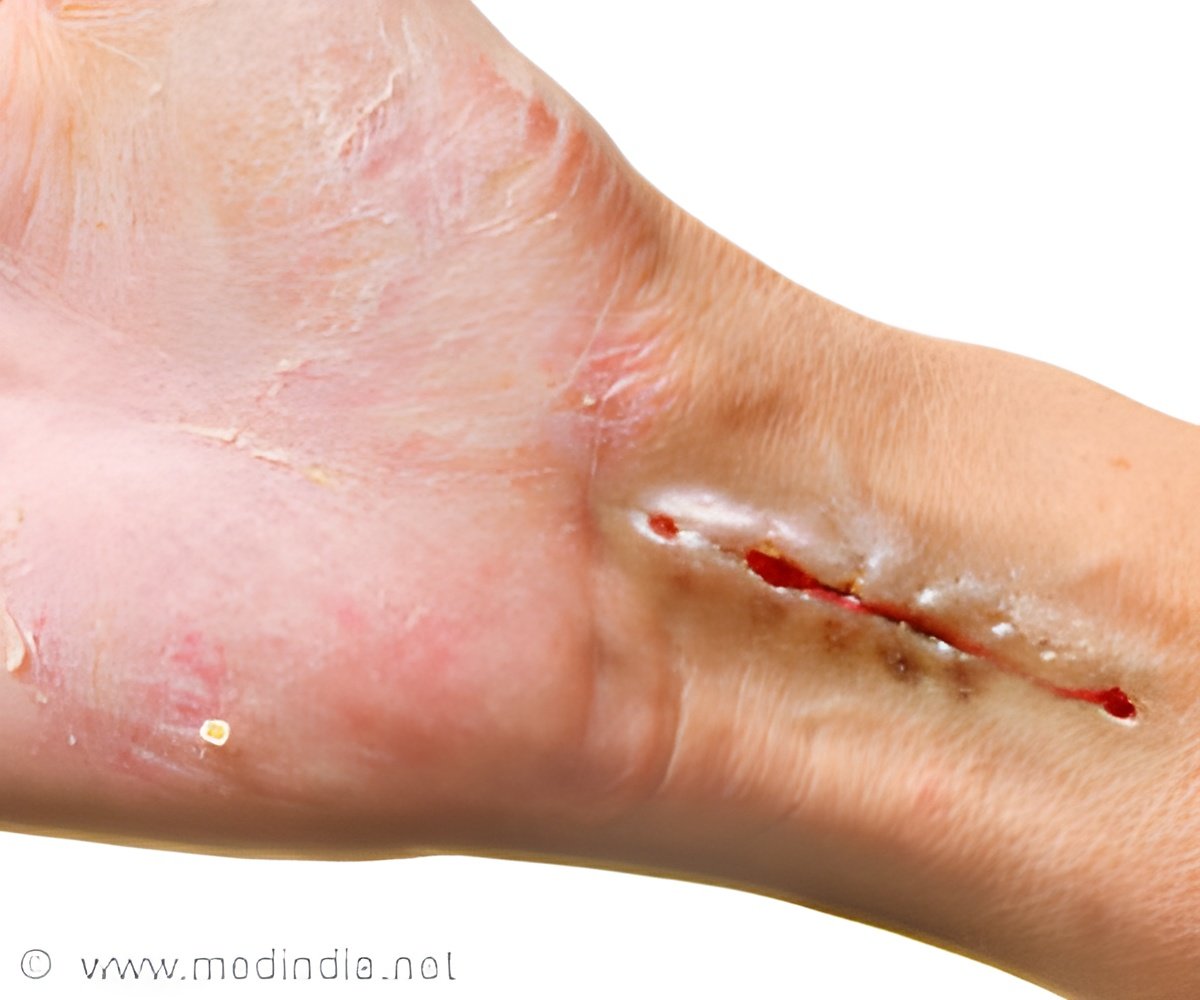
‘New study findings are important for health care providers, as existing WHO and NICE guidelines have significant cost implications for countries with limited resources.’
Read More..




But, the world’s largest wound infection trial could not show superiority of these interventions over cheaper alternatives.Read More..
Publishing their findings in The Lancet, researchers participating in this study are calling for guidelines recommending either specifically to Low- and Middle-income Countries (LMIC) or at a general global level, to be revised.
Mr. Aneel Bhangu, from the University of Birmingham, commented: “Surgical site infection is the world’s most common postoperative complication - a major burden for both patients and health systems.
We have delivered the biggest trial of its kind, where we could not demonstrate the superiority of these interventions over cheaper alternatives.
“Our findings are hugely important for a wide range of care providers in LMICs, as following existing WHO and NICE guidelines, which have significant cost implications for organizations which have limited resources.”
Advertisement
Those patients in LMICs are disproportionately affected by higher rates of SSI compared to those in high-income countries - increasing the risk of catastrophic expenditure, impoverishment, and wider negative community impact.
Advertisement
Professor Adesoji Ademuyiwa, from the University of Lagos, commented: “The overall SSI rate was very high at 22% - a preventable complication that is causing unnecessary suffering and burden to patients and systems.
“It is clear that small randomized trials should now be avoided and should be replaced with larger trials that can provide more robust evidence on the incidence of SSI, ultimately leading to more effective measures to help tack this global healthcare challenge.”
Source-Medindia