Newly identified pathway could be a therapeutic target for halting the fibrosis process and for restoring organ function, says study.
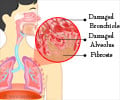
Normal healing requires the interaction of many cell types - immune cells, stem cells, and the connective tissue cells called fibroblasts. Activated fibroblasts are called myofibroblasts and orchestrate many aspects of the healing process. But severe or repetitive injury can cause myofibroblasts to become hyperactive, setting off an out-of-control tissue buildup that leads to fibrosis. The molecular mediators underlying fibroblast activation are poorly understood, and their identification was a primary focus of the current study.
Study Overview
"We specifically wanted to identify factors that could control profibrotic activity without affecting normal wound healing," says co-corresponding author Mohit Kapoor, PhD, director of Arthritis Research in the Krembil Research Institute at UHN. "Identifying these factors will not only further enhance our understanding of the pathogenesis of fibrosis but will also provide new antifibrotic targets for multiple diseases."
The investigators first searched a gene expression database for genes that were upregulated in fibroblasts from patients with two important fibrotic diseases - idiopathic pulmonary fibrosis (IPF) and systemic sclerosis or scleroderma - compared with healthy individuals. One of the genes they identified codes for the cell membrane protein ephrin-B2, which binds to receptors on adjacent cells. During normal development ephrin-B2 regulates the formation and assembly of blood vessels, and its activity has been associated with the development of new blood vessels within and around malignant tumors.
After confirming increased ephrin-B2 expression in a set of lung fibroblasts from patients with IPF, the team found that suppressing ephrin-B2 expression blocked the development of lung fibrosis in a mouse model. Further investigation revealed that the attraction of additional fibroblasts to sites of lung injury and their activation into myofibroblasts was set off when the portion of the ephrin-B2 protein that extends outside the cell membrane was clipped off by an enzyme called ADAM10, releasing a molecule called sEphrin-B2.
Advertisement
In their mouse model of lung fibrosis, the inhibition of ADAM10 prevented the shedding of sEphrin-B2 and reduced fibrosis and death. Increased ADAM10-sEphrin-B2 signaling was also found in lung fibroblasts from IPF patients, and the enzyme's inhibition reduced sEphrin-B2 shedding in cells from both IPF patients and healthy volunteers. Suppression of ADAM10-sEphrin-B2 signaling also reduced the expression of several fibrosis-associated proteins in myofibroblasts from patients with IPF, and levels of sEphrin-B2 were higher in both lung fluid and plasma from patients than from healthy volunteers.
Advertisement
A guiding force behind this work was the late Andrew Tager, MD, of the MGH Pulmonary and Critical Care Medicine, who founded what is now the Andrew Tager Fibrosis Research Center. "Andy was a giant in pulmonary fibrosis research and one of the most influential scientific minds in the field. His vision and insight were crucial to the discovery of the ADAM10-sEphrin-B2 pathway in pulmonary fibrosis" says Lagares. A co-author of this Nature Medicine paper and an associate professor of Medicine at Harvard Medical School, Tager passed away in August 2017.
Source-Eurekalert