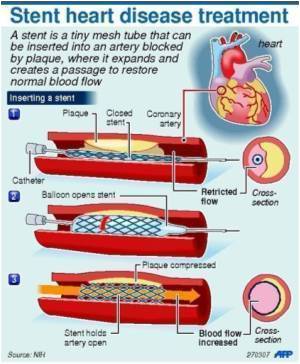
Results from the SCAAR study, presented at the ESC Congress 2011 today, showed that Percutaneous Coronary Intervention (PCI) with new generation Drug Eluting Stents, was
The purpose of this study was to evaluate the long-term outcome in all patients who underwent stent implantation with bare metal stents (BMS), "older generation" drug eluting stents (o-DES), and "new generation" drug eluting stents (n-DES) in Sweden, using a national registry with complete consecutive enrolment, the Swedish Coronary Angiography and Angioplasty Registry (SCAAR).
The SCAAR holds data on consecutive patients from 29 centers that perform coronary angiography and percutaneous coronary intervention (PCI) in Sweden. The registry is sponsored by the Swedish Health Authorities and is independent of commercial funding. The technology is developed and administered by the Uppsala Clinical Research Center. Since 2001, SCAAR has been Internet-based, with recording of data online through an Internet interface in the catheterization laboratory; data are transferred in an encrypted format to a central server at the Uppsala Clinical Research Center.
All consecutive patients undergoing coronary angiography or PCI are included. Information with respect to restenosis and stent thrombosis has been registered for patients undergoing any subsequent coronary angiography for a clinical reason since the beginning of 2004.
Our study included 94384 stent implantations in Sweden (BMS, n=64631; o-DES, n= 19202; n-DES, n=10551), from November 2006 to October 2010. Follow-up was performed up to two years post-intervention.
The performance up to two years of different types n-DES was evaluated in an unselected large real-world population- including patients with myocardial infarction, three-vessel and/or left main disease, bifurcation lesions, graft disease, restenotic lesions and chronic total occlusions.
Advertisement
Improved stent designs with thinner struts, more biocompatible polymers may have an important impact on drug elution profiles, endothelial coverage, and functional recovery.
Advertisement
These findings can be useful for the management of patients with high risk profile that could benefit more from these new devices.
Source-Eurekalert