Results from a prospective clinical utility study of its Myriad myPath Melanoma test were presented by Myraid Genetics.
Results from a prospective clinical utility study of its Myriad myPath Melanoma test were presented by Myraid Genetics at the 2014 American Society of Dermatopathology (ASDP) annual meeting in Chicago, Ill.
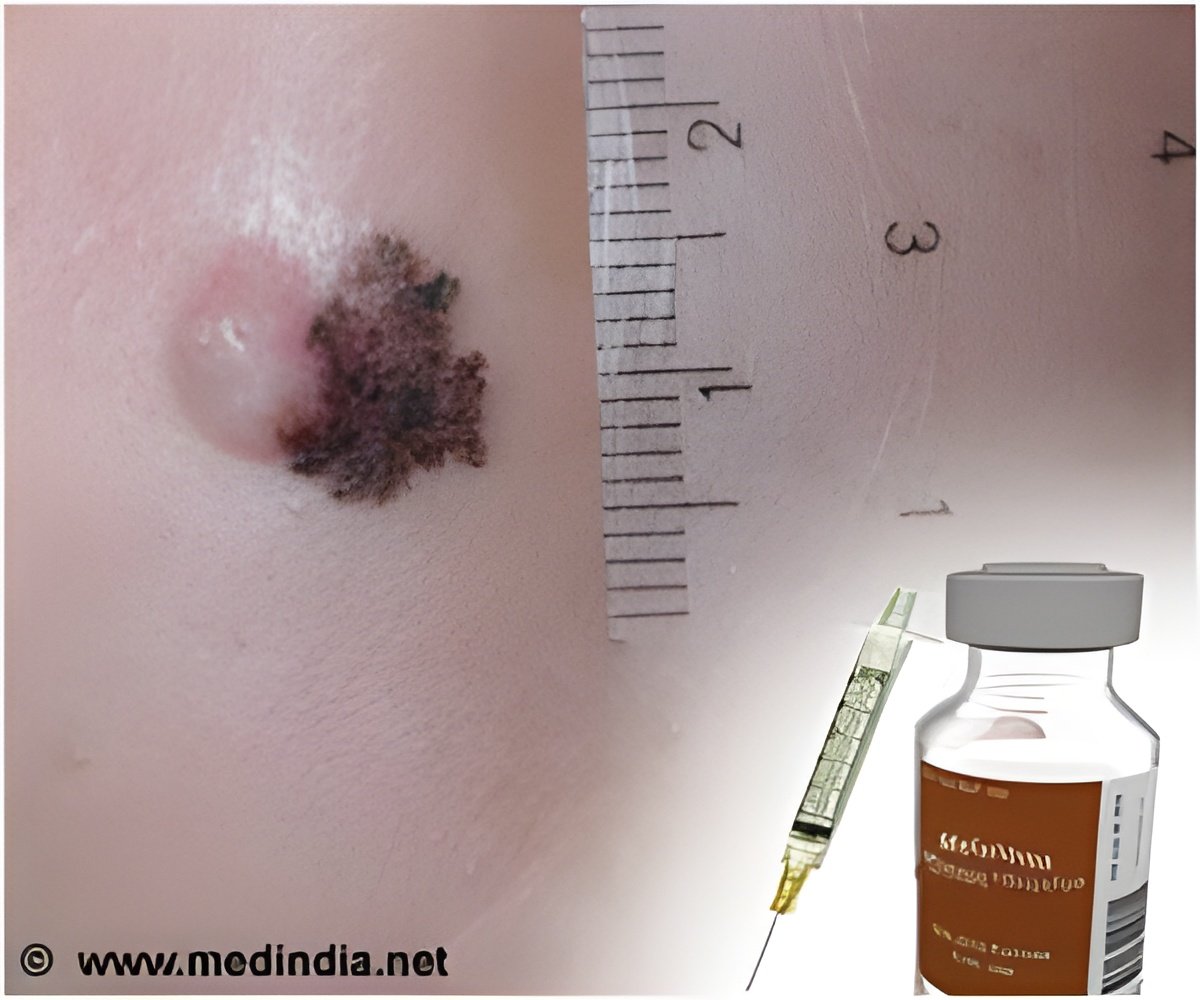
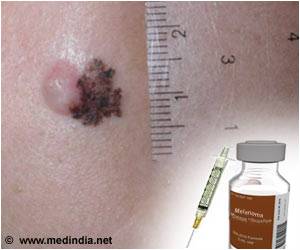
Myriad myPath Melanoma is a genetic test that differentiates malignant melanoma from benign skin lesions across all major melanoma subtypes. Key findings of this clinical utility study included a 43 percent reduction in indeterminate diagnoses and a 49 percent change in physicians' treatment recommendations for patients.
"These findings demonstrate the power of Myriad myPath Melanoma to improve patient care through more definitive diagnoses of skin lesions, particularly in these difficult-to-call cases," said Loren Clarke, M.D., vice president of Medical Affairs at Myriad Genetic Laboratories. "Importantly, the number of indeterminate cases was significantly reduced, which means less uncertainty for more patients and physicians, and may lead to less overtreatment in these cases. "The study evaluated the impact of the Myriad myPath Melanoma diagnostic test on dermatopathologists' diagnoses and intended treatment recommendations for 218 patients with pigmented skin lesions that were considered difficult to diagnose. The dermatopathologists recorded their diagnoses and treatment plans before and after receiving the myPath Melanoma test results. The changes in patient diagnoses are summarized in the table below.
| Pathology Diagnosis | Pre-Test (N=218) | Post-Test (N=218) | % Change |
| Benign | 10.6% | 40.8% | +30.2% |
| Malignant | 9.2% | 21.6% | +12.4% |
| Indeterminate | 80.3% | 37.6% | - 42.7%
|
The dermatopathologists also were asked how the Myriad myPath Melanoma test result would change their intended treatment recommendations for patients. Overall, changes in treatment recommendations were observed in 49.1 percent of difficult-to-diagnose cases. In 39.4 percent of patients receiving a benign test result, recommendations were downgraded to less invasive treatment. Conversely, in 45.8 percent of patients receiving a malignant test result, recommendations were upgraded to more invasive treatment. "These data strongly support the integration of the Myriad myPath Melanoma test into clinical practice to personalize and improve patient care," said Clarke. "The Myriad myPath Melanoma test objectively answers a vital clinical question for physicians: Does my patient have malignant melanoma that requires aggressive intervention, or a harmless skin lesion that should be monitored?"
Source-Eurekalert