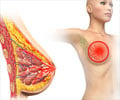
The natural switch between the body's inflammatory response and how malignant breast cancer cells use the bloodstream to spread has been identified by Cornell researchers.

The laboratory of Michael R. King, Cornell professor of biomedical engineering has developed a flow chamber that mimics an inflamed endothelium (the blood vessel wall) and has used this to investigate the metastatic cascade.
In understanding the adhesive behavior of a particularly metastatic cell line, King and Yue Geng, graduate student in the field of biomedical engineering, discovered unexpectedly that these cells were unable to interact with selectins (receptor sites on the endothelium) - a key step in the metastatic cascade. This mechanism is identical to how white blood cells infiltrate blood vessels to reach the site of inflammation.
Cancer has long been associated with inflammation - the body’s natural defense mechanism - and now the researchers have demonstrated a definitive link. They found that the presence of pro-inflammatory molecules - the cytokines IL-6 and TNF-alpha - enable the malignant, hormone therapy-resistant breast cancer cells used in the study to adhere to the endothelial wall, leading to metastasis.
Before the cancer has spread, tumor cells first encounter IL-6 and TNF-alpha in the primary tumor’s microenvironment. These cytokines induce proliferation and aggregation of cancer cells, triggering other cancer cells to secrete more cytokines, resulting in a positive feedback loop.
The bioengineers went on to design several different cell culture setups to culture cancer cells with human plasma, IL-6 and TNF-alpha to test their hypotheses that inflammatory molecules in blood may induce adhesion capability. All of them promoted breast cancer cell metastatic behavior.
Advertisement
Improving cancer treatment to fight metastasis via the bloodstream will depend on undoing this roll-and-stick mechanism of cancer cells, Geng says. The Cornell research could form the basis for immunotherapies to block the ligand-selectin binding of cancer cells, by first counteracting the inflammatory cytokines that, it seems, set the whole process in motion.
Advertisement
The paper is available at:
http://dx.plos.org/10.1371/journal.pone.0054959
The National Cancer Institute via the Center on the Microenvironment and Metastasis, and a National Science Foundation Graduate Research Fellowship, funded the research.
Source-Newswise