Psoriasis patients who have been long-awaiting new options in treatment, now have fresh hope with the Food and Drug Administration's approval of the
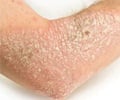
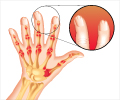
Psoriasis patients who have been long-awaiting new options in treatment, now have fresh hope with the Food and Drug Administration's approval of the new drug, Amevive. This unique drug targets renegade immune cells to control hard-to-treat psoriasis. Psoriasis has been thought to occur when the immune system goes amok and cells called memory effector T cells prompt skin inflammation. The treatments that are currently available for psoriasis range from ointments to ultraviolet therapy to other injected drugs that can broadly suppress the immune system. The new drug Amevive, however has been designed to target only the harmful T cells, instead of the entire immune system. Physicians have been eagerly awaiting the drug as it works differently from those drugs that are available in the market presently.
The drug, whose effect has not yet been compared with existing treatments, will be marketed in intravenous and intra-muscular injection forms. The treatment, for a 12-week course, will cost between $7000 and $10,000 and the weekly dose will be administered under the supervision of a doctor.Biogen Inc., the makers of the drug, sponsored a study, which involved patients with chronic psoriasis that covered at least 10 percent of their bodies, and gave them either Amevive or dummy injections. They found that 40 per cent of patients on the Amevive drug saw their psoriasis symptoms cut in half, as compared to about 10 percent of placebo-treated patients. A small number of patients even saw their lesions almost vanish, for at least a while. Though the duration for which the effect lasted was not known, a small subset of patients went seven months or longer before needing additional treatment. The FDA's Dr. Karen Weiss cautioned that though the drug has a much more selective effect, it is still too soon to say it is very selective and very safe. In fact, she added that patients on the drug would require a blood test before each weekly dose to ensure their levels of infection-fighting immune cells have not dipped too low, which would require postponing a dose. Another theoretical concern is that by suppressing the immune system, the risk of cancer might increase. The FDA, while not restricting the number of times patients can repeat Amevive therapy when their psoriasis recurs, has warned that the effects of the drug on developing fetus is not yet known.