According to researchers, Americans will probably have to depend on current anthrax vaccines if there is another such biological attack this year
According to researchers, Americans will probably have to depend on current anthrax vaccines if there is another such biological attack this year Second-generation vaccines are in the works but not ready. There is only one approved anthrax vaccine that's ready to go, approved by the FDA.
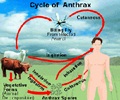
The government last week cleared the nation's sole maker of the anthrax vaccine to resume shipping after four years in which its factory repeatedly failed FDA inspections.``This is the only vaccine that we will have if there is another anthrax attack,'' Lucey said. Last fall's anthrax outbreak killed five people on the East Coast. The Washington Hospital Center tested more than 400 people after anthrax was discovered in the Washington area, but no positive cases were discovered.The anthrax vaccine requires a series of six shots over 18 months and must be given before exposure. Under limited circumstances, doctors can apply the vaccine after exposure to anthrax, a regimen that only requires three shots over one month. ``Yes, it's an FDA-licensed vaccine, but it was licensed to be given before exposure to anthrax,'' Lacey said.
There are other promising areas of research, Lucey said, including a protective protein culled from the blood of soldiers who received the anthrax vaccine.The FDA would have to consent before doctors could try the immune globulin on any patients, but using this kind of immune system cell as therapy has worked for other diseases, Lucey said. Immune globulin wouldn't replace antibiotic treatment. But antibiotics kill only anthrax bacteria, not the cell-killing toxin those bacteria produce in people's blood. The hope is that immune globulin would neutralize the toxin.