A team of scientists has developed a human monoclonal antibody that neutralizes the Hepatitis C virus.
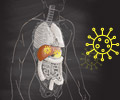
Taking aim at a leading cause of liver failure in the United States, a team of scientists at the Massachusetts Biologic Laboratories (MBL) of the University of Massachusetts Medical School (UMMS) has developed a human monoclonal antibody that neutralizes the Hepatitis C virus (HCV). The new antibody effectively neutralized the virus in culture, and then prevented infection by the virus in a pre-clinical animal model of the disease.
Details of the research were presented April 23 in Copenhagen, Denmark at the 44th Annual Meeting of the European Association for the Study of the Liver (EASL). "We are pleased with the progress of this program," said Donna Ambrosino, MD, executive director of the MBL and a professor of pediatrics at the Medical School. "This antibody shows significant efficacy against the virus."In the current study, MBL scientists injected transgenic mice (HuMAb Mouse® technology, Medarex, Inc.) with elements of HCV and then painstakingly searched for individual human antibodies produced in the mice that would recognize and bind to the HCV's outer coat, known as the glycoprotein. Once they found human antibodies that looked promising, they evaluated in vitro the ability of those antibodies to neutralize the virus and selected a lead candidate antibody for further characterization. Collaborative work with clinical researchers from the Department of Medicine at the Medical School's Worcester campus demonstrated that this antibody, now known as MBL-HCV1, was able to bind tightly with all genotypes of HCV tested from infected patient samples.
MBL-HCV1 was then tested off-site on three non-human primates. In that study, one animal received no antibody, one a low dose of the new antibody, and one a higher dose. Then all three animals were exposed to HCV. The animals with low or no antibody dosages developed HCV infections, but the animal with the higher dose was protected. Subsequently, researchers gave the high-dose of the antibody to the animal that originally received no antibody, and in that case the HCV was cleared from that animal's system. "These results are encouraging as a possible treatment for HCV infected patients, but more work needs to be done before we know how effective it will be in people," Dr. Ambrosino noted.
HCV attacks the liver and can eventually lead to liver failure. According to the U.S. Centers for Disease Control and Prevention, 3.2 million Americans are chronically infected with HCV and some 10,000 die annually of the disease. Globally, as many as 170 million people are estimated to suffer from HCV infection. For the most serious cases of HCV that do not respond to antiviral drugs, liver transplantation is the only option.
Typically 2,000 to 4,000 liver transplants are done each year in the United States (far less than the number of people on the waiting list for available organs). Transplantation can be a life saving treatment; however, in nearly all cases the patient's new liver is eventually infected by HCV because the virus remains in the patient's bloodstream during surgery. The powerful antiviral drugs now used to attack HCV prior to end-stage liver failure are not routinely used during surgery due to the patients' weakened condition and because of the strong medication used to avoid rejection of the new liver. After re-infection with HCV, nearly 40 percent of patients suffer rapid liver failure.
To close that clinical gap, the new antibody developed at MBL is designed to be a therapy shortly before and after transplant surgery. By giving a patient the new antibody before and during the time when the donor liver is implanted, researchers hope the HCV virus left in the bloodstream will be neutralized and rendered unable to infect the new liver. Then, because monoclonal antibodies are highly specific and typically have little or no side-effects, additional dosages of the new antibody could, theoretically, be given immediately after transplant surgery to continue neutralizing any remaining virus.
Advertisement
Source-Eurekalert
SRM