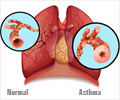
Fevipiprant (QAW039), a new asthma pill, has the power to significantly reduce the severity of the condition, revealed a study led by the University of Leicester.

‘Fevipiprant (QAW039), a new asthma pill, has the power to significantly reduce the severity of the symptoms, improve lung function, reduce inflammation and repair the lining of airways.’





It significantly decreased the symptoms of asthma, improved lung function, reduced inflammation and repaired the lining of airways. The research was funded by Novartis Pharmaceuticals, National Institute for Health Research (NIHR) and the EU (AirPROM), and is described by the lead researcher, Professor Chris Brightling, NIHR Senior Research Fellow at the University of Leicester, as "a game changer for future treatment of asthma." The drug is currently being evaluated in late stage clinical trials for efficacy in patients with severe asthma, according to ClinTrials.gov.
A total of 61 people took part in the research. One group was given 225mg of the drug twice a day for 12 weeks and the other participants were assigned to a placebo group. Fevipiprant and the placebo were added to the medications the participants were already taking.
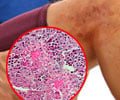
The study was designed primarily to examine the effects on inflammation in the airway by measuring the sputum eosinophil count.
The sputum eosinophil is an inflammation measurement of a white blood cell that increases in asthma and is used to assess the severity of this condition. People who do not have asthma have a percentage of less than one and those with moderate-to-severe asthma typically have a reading of about 5%.
Advertisement
Professor Christopher Brightling, who is a NIHR Senior Research Fellow and Clinical Professor in Respiratory Medicine at the University of Leicester, led the study at the NIHR Respiratory Biomedical Research Unit, which is based at the Glenfield Hospital in Leicester.
Advertisement
Gaye Stokes from Grantham in Lincolnshire has had severe asthma for 16 years. She took part in the trial and was part of the Fevipiprant group.
The 54-year-old said, "I knew straight away that I had been given the drug. I felt like a completely different person. I had more get up and go, I was less wheezy and for the first time in years I felt really, really well. For me, it felt like a complete wonder drug and I can't wait for it to be available because I really think it could make a huge difference to me."
After the 12 week trial and Gaye stopped receiving the drug, she said her health started to "go downhill again very quickly".
Professor Brightling added that the latest advance underpinned the work of the Leicester Precision Medicine Institute, a Center of Excellence that coalesces and aligns the research missions of the University of Leicester and the NHS in Leicester.
Future treatment of human disease will increasingly move from a 'one size fits all' approach to one of tailoring the treatment to the individual patient.
The NIHR Leicester Respiratory Biomedical Research Unit - a partnership between the University of Leicester and Leicester's Hospitals - focuses on promoting the development of new and effective therapies for the treatment of respiratory diseases including severe asthma and chronic obstructive pulmonary disease (COPD).
Source-Eurekalert