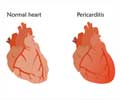
Cleveland Clinic researchers leading a global clinical trial have found that Rilonacept, an FDA approved drug for other inflammatory diseases, resolved acute pericarditis episodes and reduced risk of pericarditis recurrence.
‘Recurring pericarditis is painful and can be debilitating. Rilonacept proved its safety in pericarditis through the Rhapsody trial.’
Read More..




Pericarditis can be acute, recurrent or chronic, and often occurs after a viral infection or cardiac surgery. Recurrent pericarditis usually occurs 4-6 weeks after the first episode of acute pericarditis and often causes debilitating chest pain, physical limitations, hospitalizations and decreased quality of life.Read More..
Currently, there are no FDA approved therapies for pericarditis. Anti-inflammatories and steroids- often with harsh side effects- are used to treat the condition. The Rhapsody global Phase 3 clinical trial studied 61 patients with recurrent pericarditis, who were randomized to rilonacept or placebo.
After 16 weeks of treatment, 81% of patients on once-weekly rilonacept, reported no or minimal pericarditis symptoms versus 25% on the placebo. The drug not only resolved active episodes after the first dose, but it also decreased recurrences by 96%.
All patients involved who had been taking corticosteroids tapered and successfully transitioned to rilonacept.
"Recurring pericarditis is painful and can be debilitating to those who suffer from it," said Allan Klein M.D., director of the Center for the Diagnosis and Treatment of Pericardial Diseases at Cleveland Clinic, co-principal investigator of the study, and a paid member of Kiniksa Pharmaceuticals' scientific advisory board.
Advertisement
Of the 25 safety events in the study, 23 occurred in the placebo group, while only two occurred in the rilonacept group.
Source-Eurekalert