In people in ulcerative colitis, mirkizumab showed positive results, stated study.
Mirikizumab: First Approval
Go to source). Around the world, it is estimated that millions live with ulcerative colitis (2✔ ✔Trusted Source
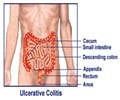
Ulcerative colitis
Go to source), a condition that is only growing in prevalence. Geert D’Haens, lead author and Professor of Gastroenterology at Amsterdam UMC, says “there is still a high unmet need for safe and effective treatments for ulcerative colitis. This new medicine meets this need for an important proportion of patients.”
What is Ulcerative Colitis
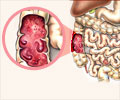
Ulcerative colitis is a common chronic disease of the colon. Patients have bloody diarrhea, abdominal pain, anemia and fatigue. Many patients have an impaired quality of life and current treatments cannot always control the disease. In that case, patients need to undergo surgery (colectomy) with a stoma or a pouch-construction. Chronic inflammation of the colon is also associated with an increased risk of cancer.‘Mirkizumab, an interleukin-23p19 antagonist was found to double the rate of remission (50%) in patients with ulcerative colitis. #digestivedisorder #clinicaltrials’





Researchers identified Interleukin-23 as a very important protein in triggering and maintaining gut inflammation, both in Crohns disease and ulcerative colitis and also in the chronic skin disease psoriasis. Previously it has been shown that blocking interleukin-23 with specific therapeutic antibodies is highly effective for those with psoriasis and Crohn’s disease. Mirikizumab is the first such antibody that was tested for ulcerative colitis. In recent years, a large group of researchers from around the globe performed two Phase 3 clinical trials to assess the safety and efficacy of mirikizumab in 1281 adult ulcerative colitis patients with moderate to severe inflammation. For comparison, a “control group” of comparable ulcerative colitis patients was not treated with mirikizumab but with a placebo. Patients received 300 mg mirikizumab or placebo (in a 3:1 ratio) via an infusion every 4 weeks for 12 weeks in total (LUCENT-1 study). If patients responded to mirikizumab in these 12 weeks (544 out of 1281 patients), they continued in the LUCENT-2 study where they received 200mg mirikizumab or placebo (in a 2:1 ratio) via an injection every 4 weeks for 40 additional weeks.
Ulcerative Colitis: Clinical Trials
Patients who were treated with mirikizumab were more likely to achieve clinical remission at both the end of the LUCENT-1 and LUCENT-2 than patients treated with placebo (LUCENT-1 24.2% versus 13.3% and LUCENT-2 49.9% versus 25.1%). The patients that received mirikizumab also had higher clinical response, endoscopic remission, and less bowel movement urgency. Interestingly, mirikizumab treatment appeared very safe. Adverse events were not more common with mirikizumab than with placebo treatment.References:
- Mirikizumab: First Approval - (https://pubmed.ncbi.nlm.nih.gov/37389706/)
- Ulcerative colitis - (https://www.mayoclinic.org/diseases-conditions/ulcerative-colitis/symptoms-causes/syc-20353326)
Source-Eurekalert