Researchers in New Zealand and Australia have collaborated to develop a new gel to heal wounds after sinus surgery.
Researchers in New Zealand and Australia have collaborated to develop a new gel to heal wounds after a sinus surgery.
Emeritus Professor Brian Robinson of Otago’s Chemistry Department produced the gel; Professor Peter-John Wormald of the University of Adelaide and ENT surgeon Mr Simon Robinson of Robinson Squidgel Ltd, performed the clinical trials.The gel, derived from a polymer named chitosan extracted from crab-shell and squid, has undergone successful sheep and human trials over the past four years. It could potentially lead to a reduction in the number of post-operative complications which frequently occur following sinus surgery.
Leading United States-based medical technology company Medtronic have purchased the patent for the gel application.
Work began on the project five years ago after Professor Robinson’s son and ENT surgeon Mr Simon Robinson challenged his father to come up with a biological substance to help solve the disheartening problem of post-operative complications after sinus surgery.
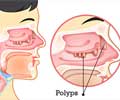
Mr Robinson says the ‘adhesions’ or scarring can block sinus passages, often requiring further surgery to correct. This affects roughly one third of all sinus-related operations.
He says the new gel is inserted into the nasal passage and forms a coating over the wound so that adhesions cannot form, and it also helps to stop bleeding with its superior blood clotting properties.
Advertisement
He believes the gel has potential to be adapted with further research for other surgical procedures, such as for brain and abdominal surgery. Both he and Professor Robinson are hopeful of continuing the research to find other applications. The former Foundation For Research, Science and Technology has funded Otago’s part in the research to date.
Advertisement
“From a research perspective, a commercialisation opportunity of this size is a clear reflection of the high calibre of the science conducted at Otago in collaboration with the University of Adelaide,” she says.
“From a financial perspective, the deal will create new opportunities because a large proportion of the proceeds will be used to support additional research and to foster future commercialisation activity. This University has extensive research expertise in basic biomedical science and we look forward to additional deals of this kind in the near future.
“Last, but certainly not least, the gel that these researchers have developed will reduce bleeding and suffering for patients undergoing ENT surgery, which is what motivated the research in the first place.”
She says commercialisation of intellectual property of this kind can be a challenging part of the business.
“The success of this particular deal is due to a combination of factors including outstanding science, clinical expertise, commercialisation experience and research funding. It has taken a small army of highly talented individuals to make the gel a commercial reality. ”
According to researchers, the new gel has the potential to assist in some half a million endoscopic sinus operations to relieve sinusitis performed each year in the US alone. In New Zealand, the figure is several thousand.
Professors Robinson, Lyall Hanton, Jim Simpson and a team of Otago researchers were part of the original team which began looking at how to exploit the properties of chitosan for use in surgery.
The Otago group initially found a way of making the chitosan water soluble. Dr Zheng Shi in the group then made a critical discovery that by using a starch-like chemical with the chitosan, they could produce a new type of natural biomedical gel.
The team was then expanded to include clinicians Mr Robinson and Professor Wormald, a world leader in ENT research and polymer expert Assoc Prof Steve Moratti, to exploit the discovery. Sheep trials and finally human trials over the past few years showed that the gel had excellent anti-adhesion and haemostatic properties.
“There was a tremendous amount of work to get the gel to this stage. We are delighted to help facilitate the transfer of great science at Otago to a good home in Medtronic, which has strong clinical and distribution capabilities,” says CEO of Otago Innovation Ltd Colin Dawson.
A wholly–owned subsidiary of the University of Otago, Otago Innovation Limited is charged with responsibility for the commercialisation of intellectual property arising from research within the University.
Source-Medindia