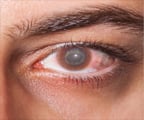
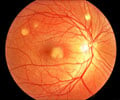
New gene therapy can help treat inherited retinal dystrophy, a rare genetic disorder caused by RPE65 gene mutations which eventually causes blindness.

‘Luxturna is the first therapy option for the hereditary retinal dystrophy
with mutations of the RPE65 gene.’





It is a severely debilitating disease, characterized by a progressive loss of vision. Most patients will be blind by the time they are young adults. There is currently no treatment for this disease; support to patients is limited to measures allowing the management of the disease such as aids for low vision.Luxturna is meant for patients with confirmed biallelic mutations of the RPE65 gene (i.e. patients who have inherited the mutation from both parents) and who have sufficient viable retinal cells.
It is the first gene therapy to be recommended for approval for a retinal disease. Luxturna works by delivering a functional RPE65 gene into the cells of the retina through a single retinal injection, which restores the production pathway for the required enzyme thereby improving the patient's ability to detect light.
Luxturna was studied in 41 patients. In the main clinical trial supporting the approval of Luxturna, patients treated with the medicine showed a significant improvement of night vision, one of the typical symptoms of the disease, after one year, while no improvement was seen in the control group. The most common side effects were conjunctival hyperaemia (eye redness), cataracts and increased intraocular pressure .
Given the novelty of the treatment and the limited number of treated patients, the CHMP requires the company to ensure the long-term follow-up of patients to confirm Luxturna's continuing efficacy and safety. Follow-up studies were agreed, including a post-authorization safety study (PASS) based on a disease registry in patients with vision loss due to inherited retinal dystrophy caused by confirmed biallelic RPE65 mutations, as well as a 15-year follow-up programme of efficacy and safety outcomes for all patients treated in the clinical programme.
Advertisement
The opinion adopted by the CHMP is an intermediary step on Luxturna's path to patient access. The opinion will now be sent to the European Commission for the adoption of a decision on an EU-wide marketing authorization . Once the marketing authorization has been granted, decisions about price and reimbursement will take place at the level of each Member State, taking into account the potential role/use of this medicine in the context of the national health system of that country.