A new genetic mutation could be the cause of childhood blindness in children as young as 3 years old.
- Glaucoma is one of the leading causes of blindness in the world
- It affects up to 5% of children worldwide
- Recent research has found that mutation in a gene could be the root cause of childhood glaucoma
Genetic testing can be done to identify children in a family who may be at risk for the disease and start disease surveillance and conventional treatments earlier to preserve their vision.
Congenital glaucoma, also known as childhood glaucoma, is a rare but serious disease that presents in children as early as birth and as late as 3 years of age. Despite its rarity, childhood glaucoma is responsible for 5 percent of cases of child blindness worldwide.
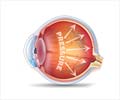
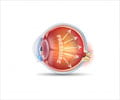
Glaucoma causes irreversible damage to the eye’s optic nerve, often due to built up pressure inside the eye (intraocular pressure, or IOP). In adults, this damage can occur over time without symptoms.
Children and babies with childhood glaucoma, however, can be born with severe disease and vision loss or lose their vision later in childhood due to elevated IOP. This increase in pressure not only damages the optic nerve but can also affect other structures like the cornea. Children with childhood glaucoma might require surgeries as early as the first three to six months of their lives, followed by several more operations throughout their childhood.
Genetics and Childhood Glaucoma
For years, researchers have turned towards genetics to better understand the cause of glaucoma. When research started, scientists were only able to identify regions of the genome affected by glaucoma. Thanks to advances in genomic technology, researchers now have the ability to review the complete genetic makeup of individuals with and without glaucoma to determine which specific genetic mutations play a role in the disease. The current research used a dataset of more than 34,000 adults with glaucoma to identify 127 genes associated with the condition (1✔ ✔Trusted SourceThrombospondin 1 missense alleles induce extracellular matrix protein aggregation and TM dysfunction in congenital glaucoma
Go to source).
To better study the genetic mutations in childhood glaucoma, researchers first looked at exome sequences from an American family of European-Caucasian descent who had been part of an earlier research project and found a striking and novel variant in thrombospondin-1, a well-known protein in the body involved in a number of important biological processes, such as the formation of new vessels (angiogenesis) and tissues. This mutated gene was not found in people without childhood glaucoma or in large population genetic databases. The amino acid altered by the mutation was evolutionarily conserved, indicating an important role in the protein’s function. This finding led researchers to connect with other researchers in Australia to see if they had any childhood glaucoma families with thrombospondin mutations. They surprisingly found two families with an alteration at the same amino acid: one of mixed European and Indian descent, and one Sudanese family originally from Africa.
To further test this hypothesis, the researchers collaborated with another group of researchers who developed a mouse model with the THBS1 mutation and found that the mouse also had features of glaucoma.
“Thrombospondin-1 is well known as a potent inhibitor of blood vessel growth, or angiogenesis, it was assumed at first that THBS1 mutations were disrupting blood vessel formation in the eye, but animal models showed normal angiogenesis, “said researchers.
It is seen that the mutation caused abnormal thrombospondin proteins to accumulate in the intraocular drainage structures of the eye involved with regulating IOP, which in turn, led to a build up of pressure that damaged the optic nerve and led to the loss of retinal ganglion cells, thereby causing vision loss.
This was the first time that researchers identified this kind of disease mechanism for causing childhood glaucoma.
The reason this is so important is because of the various genetic diversities present around the globe.
The new study has significant clinical implications. While more work remains before comprehensive genetic testing can be offered, every gene that is found presents another opportunity to identify causative mutations in these families through screening.
Therapeutically, knowledge of this gene mutation can lead to earlier treatments with conventional therapies. For example, if a baby is born with this mutation, their eye care specialist can better inform the parents of the risks and develop an appropriate disease-monitoring and treatment plan.
Identifying this new mechanism and gene at the root of childhood glaucoma could also lead to new therapies that target the accumulation of abnormal proteins. The researchers also aim to determine if other THBS1 mutations are involved in adult-onset diseases, like primary open-angle glaucoma, or milder forms of the disease if the mutation is not as pronounced.
The researchers will also continue to look for new genes associated with childhood glaucoma in the hopes of one day developing a more comprehensive screening.
Reference:
- Thrombospondin 1 missense alleles induce extracellular matrix protein aggregation and TM dysfunction in congenital glaucoma - (https://www.jci.org/articles/view/156967)
Source-Medindia