CDC issues updated steps or guidelines to eliminate bloodstream infection in patients with intravenous catheters.
Intended to replace the previous CDC guidelines published in 2002, the new edition was developed by a working group led by the Society of Critical Care Medicine, in collaboration with the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, Surgical Infection Society, American College of Chest Physicians, American Thoracic Society, American Society of Critical Care Anesthesiologists, Association for Professionals in Infection Control and Epidemiology (APIC), Infusion Nurses Society, Oncology Nursing Society, American Society for Parenteral and Enteral Nutrition, Society of Interventional Radiology, American Academy of Pediatrics, Pediatric Infectious Diseases Society, the Centers for Disease Control and Prevention (CDC) and the Healthcare Infection Control Practices Advisory Committee.
"The updated CDC guidelines are rich with new recommendations that are based on additional scientific research that has emerged since the prior version was published," said APIC 2011 President Russell N. Olmsted, MPH, CIC. "This is an important resource to support efforts toward the elimination of catheter-related bloodstream infections (CRBSIs)."
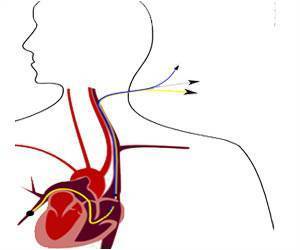
The new guide is developed for healthcare personnel who insert intravascular (IV) catheters (long, thin tubes inserted into a vein that lead to the heart), as well as persons responsible for surveillance and control of infections in hospital, outpatient and home healthcare settings.
"The goal of an effective prevention program should be the elimination of CRBSI from all patient-care areas," state the authors in the paper. "Although this is challenging, programs have demonstrated success, but sustained elimination requires continued effort. The goal of the measures discussed in this document is to reduce the rate to as low as feasible given the specific patient population being served, the universal presence of microorganisms in the human environment, and the limitations of current strategies and technologies."
A federally funded program led by renowned patient safety leader Peter Pronovost, MD, PhD, FCCM, involving intensive care units in Michigan hospitals, demonstrated the potential for the elimination of CRBSIs. The Michigan collaborative reduced the incidence of CRBSIs by two-thirds, saving more than 1,500 lives and $200 million in the first 18 months. Similarly organized initiatives in other states and countries have also demonstrated success. The critical underlying foundation for these successes has been use of five key prevention strategies from the 2002 version of CDC's guidelines that were based on published, scientific evidence.
Advertisement
"The timing for this updated guideline is perfect because starting this year hospitals that accept Medicare patients are required to report their central line-associated bloodstream infections to the Centers for Medicare & Medicaid Services, or risk losing 2 percent of their Medicare payments," said Olmsted.
Source-Eurekalert