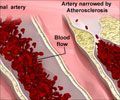
A recent study explains how gut microbes convert the nutrient carnitine into TMAO, atherosclerosis- and blood clot-promoting molecule following a red-meat-rich diet.

‘Patients who transitioned to a non-meat diet went on to exhibit reduced gut microbial levels of gbuA.’





“These new studies identify the gut microbial gene cluster responsible for the second step of the process that links a red meat-rich diet to elevated cardiac disease risks,” said Dr. Hazen, who directs the Cleveland Clinic Center for Microbiome & Human Health. “This discovery helps point us towards new therapeutic targets to prevent or reduce diet-associated cardiovascular disease risk.” In 2018, Dr. Hazen published results in the Journal of Clinical Investigation showing that dietary carnitine is converted into TMAO in the gut through a two-step, two microbe process. An intermediary metabolite in this process is a molecule called γBB (gamma-butyrobetaine).
According to Dr. Hazen, multiple gut microbes can convert dietary carnitine to γBB, but very few can transform the molecule to TMA, the precursor to TMAO. “In omnivores, Emergencia timonensis is the primary human gut microbe involved in the transformation of γBB to TMA/TMAO. Conversely, long-term vegetarians and vegans have very low levels of this microbe in their gut and therefore have minimal to no capacity to convert carnitine into TMAO.”
The researchers studied the relationship between fasting plasma γBB levels and disease outcomes using samples and clinical data collected from nearly 3,000 patients. Higher γBB levels were associated with cardiovascular disease and major adverse events including death, non-fatal heart attack or stroke.
To understand the mechanistic link between γBB and the observed outcomes in patients, the researchers studied fecal samples collected from mice and patients, as well as preclinical models of arterial injury. They found that introducing E. timonensis completes the transformation of carnitine to TMAO, elevates TMAO levels and enhances blot clot potential.
Advertisement
“By studying patient samples, we saw that the abundance of gbuA is significantly associated with a diet rich in red meat and plasma TMAO levels,” said Dr. Hazen, who is also chair of the Department of Cardiovascular Disease & Metabolic Sciences and a practicing physician.
Advertisement
The studies were performed in part during a collaboration between Dr. Hazen’s team and Procter & Gamble (P&G). Jennifer Buffa, MS; Kymberleigh Romano, PhD; and Matthew Copeland, PhD, are co-first authors on the study, which was supported in part by the National Heart, Lung & Blood Institute (part of the National Institutes of Health), the Leducq Foundation and P&G.
Dr. Hazen is named as co-inventor on pending and issued patents held by Cleveland Clinic relating to cardiovascular diagnostics and therapeutics and has the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics or therapeutics. Dr. Hazen also reports having been paid as a consultant for P&G, and receiving research funds from P&G. Dr. Hazen holds the Jan Bleeksma Endowed Chair in Vascular Cell Biology & Atherosclerosis.
Source-Eurekalert