A novel vaccine-like therapy to fight lupus has shown promising results in early European trials, say researchers.

The new treatment, developed by France-based biotechnology company Neovacs, neutralizes a protein called interferon alpha, which is seen in higher amounts in lupus patients and may be a driver of chronic inflammation and autoimmunity.
"Their data looks very interesting," said Joan Merrill, medical director of the Lupus Foundation of America, who was not involved in the research.
"It's a fascinating idea to me, that you're immunizing yourself against an element of your immune system that is overreacting," she told AFP.
"It's totally exciting. It's a novel mechanism, a novel approach to developing a new drug."
Injections of an immunotherapy called the Kinoid were shown to be safe in phase I/II trials on a small number of European patients, the company announced at the American College of Rheumatology Scientific Meeting in Chicago.
Advertisement
"The injection of the Kinoid produces a reaction of the immune system, which results in the production of various antibodies which will neutralize the alpha interferon," said chief executive Guy-Charles Fanneau de La Horie.
Advertisement
The treatment consists of a trio of injections in the first month, followed by a shot every three to four months afterward.
The randomized trial was small -- just 28 subjects -- and it did not include any non-white participants, who will also need to be studied in case they show different responses to treatment.
One patient in the trial developed a lupus flare during treatment, but was excluded from the results because it was determined she had stopped taking corticosteroid treatment and was in violation of the trial rules.
More clinical trials are on the way, and a phase IIb study could begin by the middle of next year, said Piers Whitehead, Neovacs vice president of corporate development.
"We think a key advantage of our approach is we know that we can neutralize all 13 subtypes (of interferon alpha) with our product," he said.
Researchers were also able to see changes in the gene expression profile in some patients who had too much interferon, which is a promising sign that the treatment is working, though it is far too early to call it a cure.
"That expression profile trends back towards normal and in some patients becomes normal as a response to treatment," Whitehead told AFP.
In March, the US Food and Drug Administration approved the first drug to treat lupus in 56 years.
Benlysta, developed by the US firm Human Genome Sciences and Britain's GlaxoSmithKline, targets a different protein than Neovacs's Kinoid.
Benlysta is an intravenous treatment, a monoclonal antibody, and is the first treatment to come on the US market since Plaquenil in 1955. Aspirin was approved to treat lupus in 1948.
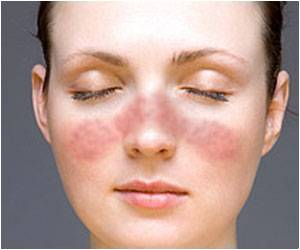
Lupus affects women of childbearing age more often than men, and is particularly common in women of color.
Five million people worldwide have lupus, which usually develops between ages 15 and 44. Its origins are uncertain, and there is no known cure.
Source-AFP