Nerivio, the first-of-its-kind product for migraine in India, holds USFDA approval, is drug-free, noninvasive, and exhibits a promising safety profile.

Dr. Reddy's rolls out Nerivio®, a USFDA-approved drug-free non-invasive migraine management device, in India
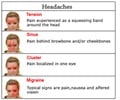
Go to source). Migraine is a global health challenge, affecting around 30 percent of adults on 15 or more days per month, impacting 1.7 to 4 percent of the population. Migraines are known to have a disproportionate impact on women, who constitute approximately 60 percent of the 213 million migraine sufferers in India alone.
‘Nerivio is a prescription-based, non-invasive device created for adults and adolescents aged 12 years and older, designed for both acute and preventive treatment of migraines. #headache #migraine #migrainetreatment #Drreddy’





The device can be worn on the upper arm. It is to be used within 60 minutes of onset of headache for acute treatment of migraine or every alternate day for prevention of migraine.The device uses the Remote Electrical Neuromodulation (REN) mechanism to specifically activate conditioned pain modulation by stimulating nerve endings. This initiates a natural pain-relieving process in the brainstem, causing a global effect of pain inhibition that affects the original source of migraine pain in the head.
Dr. Reddy's CEO on Nerivio: A Breakthrough in Migraine Management
"Nerivio is USFDA-approved, drug-free, noninvasive, first-of-its-kind product for migraine in India and offers a favorable safety profile. We believe this product meets a genuine unmet clinical need among migraine patients," said M. V. Ramana, Chief Executive Officer, Branded Markets (India and Emerging Markets), Dr. Reddy's, in a statement."The roll-out of Nerivio marks our entry into digital therapeutics.It is an area that is seeing increasing adoption by physicians as well as patients due to its potential to reduce pill burden and decrease dependency on non-specific medication in chronic or hard-to-treat diseases," he added.Nerivio is clinically proven and safe, and is suitable for a wide range of patients, including those who prefer drug-free options, individuals with contraindications to medications or poor medication tolerance, sensitive populations such as adolescents, women of vulnerable age groups and patients at risk of medication overuse headache.
Studies show that Nerivio is safe and well tolerated with no systemic side effects or concern for medication overuse. It has also proven efficacy in treating associated symptoms in migraine such as nausea, vomiting etc.Dr. Reddy's also recently signed an exclusive agreement for the commercial marketing and distribution of Nerivio in Germany, Austria, Czech Republic, Denmark, Finland, France, Italy, Norway, Poland, Slovakia, Spain, Sweden, Switzerland and the UK.
Reference:
- Dr. Reddy's rolls out Nerivio®, a USFDA-approved drug-free non-invasive migraine management device, in India - (https://www.drreddys.com/cms/cms/sites/default/files/2023-11/Press%20release%20-%20Dr.%20Reddy%27s%20Nerivio.pdf)
Source-IANS