New study covers modern approaches to the evaluation of neoplastic processes with diffusion-weighted imaging (DWI) and proposes a physical model for monitoring the primary quantitative parameters of DWI and quality assurance.
‘Early cancer diagnosis is one of the highest-priority problems for the healthcare system, because it is critical for overall treatment success and saving patients' lives.’





Such movement can be estimated through the mathematical processing of DWI data using apparent diffusion coefficient (ADC) maps. In the absence of pathological tissues, the intracellular ADC is lower compared to the intercellular ADC. However, an increase in cell density (in the presence of malignancies, especially those made up of many small-size cells) leads to a decrease in intercellular diffusion. Since ACD is not an absolute value, it makes it dependable on external factors, such as sequence and reconstruction parameters, image quality, and hardware features. In order to boost the efficacy of tumor differential diagnostics, ACD must be estimated with greater accuracy and reproducibility. This can be achieved with phantoms (also called test objects) that allow assessing the imaging quality and building various diffusion models (i.e., non-restricted, hindered, and restricted diffusion with permeable and semi-permeable membranes). Such phantom has been developed by a research team from the Innovation Technology Department of the Moscow Center for Diagnostics & Telemedicine.
The scope of application of the phantoms developed by the US National Institute of Standards and Technology, the Quantitative Imaging Biomarkers Alliance, and the Institute of Cancer Research in the UK is limited because they utilize polymer solutions. Instead, the authors of this paper suggested using a combination of siloxane-based water-in-oil (W/O) emulsions and aqueous solutions of polyvinylpyrrolidone (PVP). These components allow emulating both hindered and restricted diffusion while maintaining relatively high signal intensity. What is more, the newly-designed phantom can be used to assess the image quality control in terms of fat suppression, which again is critical for detecting pathological processes.
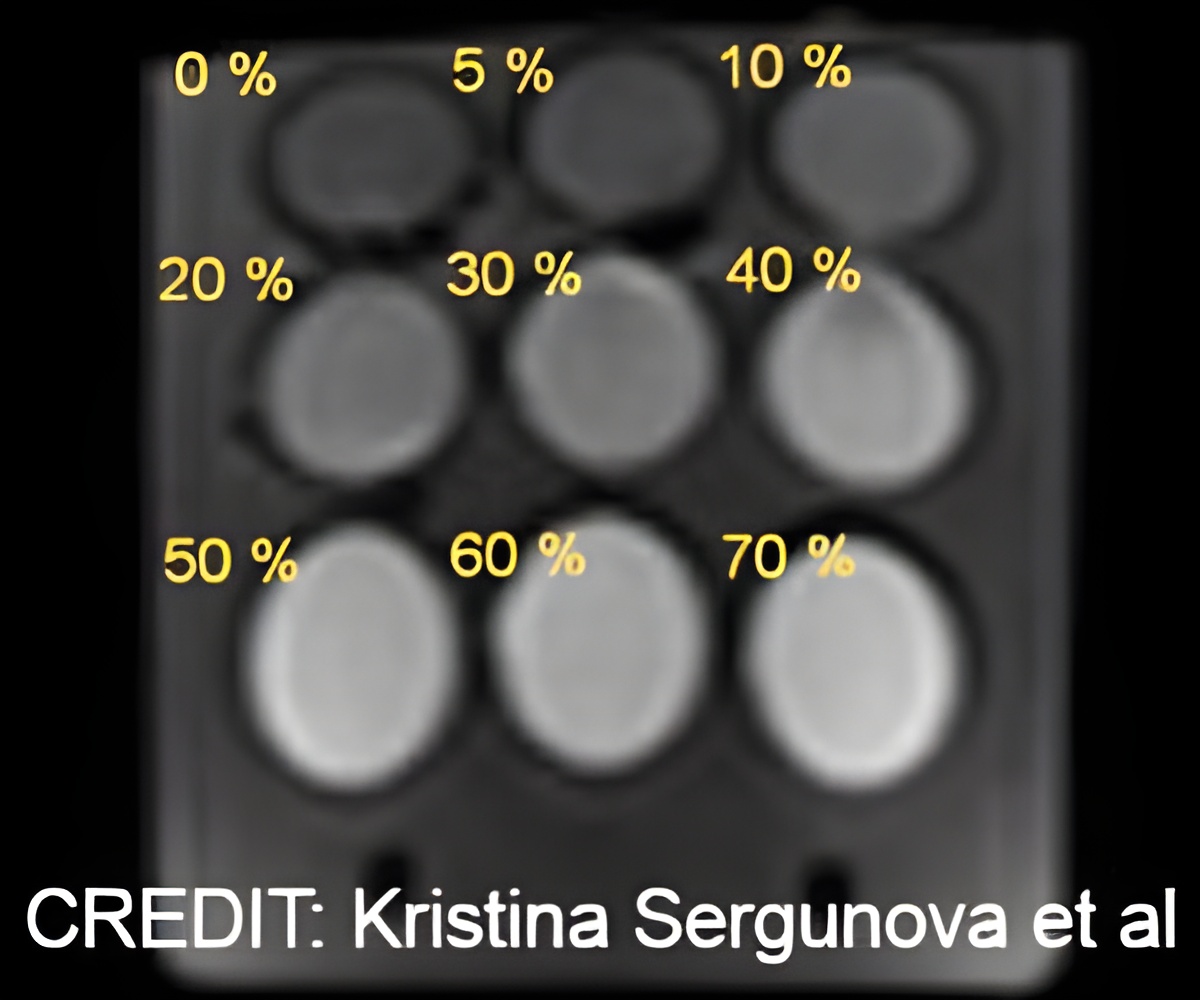
To simulate hindered diffusion, the investigators used aqueous PVP solutions with concentrations of 0 to 70%. The W/O emulsions imitated restricted diffusion in intracellular space. To attain a high DWI signal (maximum radiolucent areas), the authors incorporated silicone oil: Cyclomethicone and caprylyl-methicone. This phantom was scanned using 1.5T magnetic resonance scanner with various fat suppression techniques.
After a series of control experiments, the authors came to the conclusion that such phantom with the control substances allows modeling the apparent diffusion coefficients ranging from normal tissue to benign and malignant lesions: from 2.29 to 0.28mm2/s. Correspondingly, it is suitable for assessing the ACD measurement quality, the efficacy of fat suppression, and calibrating MR scanners from various manufacturers.
Advertisement
Source-Eurekalert