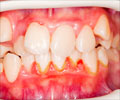
The Food and Drug Administration (FDA) has recently approved of a new prescription treatment for gingivitis, a common gum disease that affects
The Food and Drug Administration (FDA) has recently approved of a new prescription treatment for gingivitis, a common gum disease that affects almost everybody. Gingivitis is an inflammation of the gums haracterized as swollen, red, or bleeding gums. It is believed that plaque-forming bacteria that live in the mouth and on the tooth surfaces are a cause of gingivitis and substances released by the bacteria cause the gum inflammation. Reduction in the inflammation associated with gingivitis can be effectively achieved by reducing the number of plaque bacteria.
The Decapinol Oral Rinse thus works against gingivitis by reducing the number of bacteria that attach to tooth surfaces and cause dental plaque. Decapinol contains a substance called a surfactant that acts as a physical barrier, making it harder for bacteria to stick to tooth surfaces. However it is only approved for use in persons 12 years of age or older when routine oral hygiene is not adequate to prevent gingivitis and is contraindicated in pregnant women.In a comparison study conducted in adults with mild to severe gingivitis the use of Decapinol was compared either to "no treatment" or to an antimicrobial rinse. The results of the study showed that Decapinol decreased gingivitis up to 60%--compared to no treatment. Although it has proven benefits Decapinol Oral Rinse is being regulated only as a medical device and not as a drug because its primary mode of action is to create a physical barrier, rather than to act chemically.