Plug-and-play test that requires only a small blood sample to track modified T-cells in patients over time, improving the precision of cancer immunotherapy.
Duke-NUS scientists develop novel plug-and-play test to evaluate T cell immunotherapy effectiveness
Go to source).
‘Did You Know?
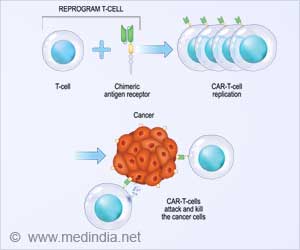
CAR T-cell therapy can sometimes cause serious or even life-threatening side effects like cardiac irregularities and seizures. Therefore, it is always administered under medical supervision and patients need to be watched closely for several weeks after getting the CAR T cells. #medindia #tcells #immunotherapy’





CAR T-cell therapy can sometimes cause serious or even life-threatening side effects like cardiac irregularities and seizures. Therefore, it is always administered under medical supervision and patients need to be watched closely for several weeks after getting the CAR T cells. #medindia #tcells #immunotherapy’
Advertisement
Adapting SARS-CoV-2 Detection Technology for Cancer Immunotherapy
T cells are immune cells that target and eliminate infected cells, including those infected by viruses, bacteria, and cancer cells. Initially developed to detect T cells specific to the SARS-CoV-2 virus, this technology has now been adapted for cancer immunotherapy. The test, which requires less than a quarter teaspoon of blood, works by stimulating the target T cells in the blood to release cytokines, chemical signals that allow clinicians to assess both the quantity and quality of the T cells involved in the immune response.Advertisement
Peptides Used to Activate Engineered T Cells
In this proof-of-concept study, which was published in Immunotherapy Advances, the research team introduced fragments, called peptides, that stimulate the T cells engineered to fight Hepatitis B virus-related liver cancer present in the treated patients. Using their test, they assessed whether the engineered T cells remained in the blood and continued to function properly after infusion into the patient.Advertisement
Making Advanced Treatments More Accessible
Assistant Professor Anthony Tan, from Duke-NUS’ Emerging Infectious Diseases Programme and first author of the study, commented:“Our innovative test enables us to swiftly detect and analyze engineered T cells in patient blood samples. Its simplicity and speed could have a significant impact on the clinical field helping to make advanced treatments more accessible.”
Growing Use of Engineered T-Cell Therapies in Cancer Treatment
With engineered T-cell therapies becoming more widely used to treat malignancies, including Hepatitis B virus-induced liver cancer and a range of blood cancers, being able to accurately and easily track how these engineered cells behave in the body over time will be crucial in monitoring the effectiveness of these therapies in individual patients.Expanding Applications: From Viral Infections to Cancer
At the same time, this plug-and-play concept can help accelerate the translation of new T-cell-based therapies from the laboratory to the patient bedside. The research team has already demonstrated that the test can be adapted for use in numerous viral infections, but this is their first foray into cancer therapies, where the test can be harnessed for T-cell receptor (TCR) engineered T cells, as well as chimeric antigen receptor (CAR) T-cell therapies.Accelerating Research in CAR and TCR T-cell Therapies
Professor Antonio Bertoletti, from Duke-NUS Emerging Infectious Diseases Programme and senior author of the study, added:“Tracking the functionality of adoptively transferred engineered T-cell products could provide important information on treatment efficacy over time, an assessment which at the moment remains largely unexplored. We hope that with this proof-of-concept, we can help accelerate research into other CAR and TCR T-cell therapies as well as support clinicians on the frontline caring for patients receiving these novel therapies.”
Evaluating the Impact of TCR T-cell Therapy in Hepatitis B
In collaboration with Lion TCR Pte Ltd, the test has been deployed in a Hepatitis B virus-TCR T-cell therapy clinical trial, called the SAFE-T-HBV trial, evaluating the effectiveness of a novel therapy in two patients and demonstrating the test’s impact on improving the precision of immunotherapy outcomes.The team is now looking to advance this proof-of-concept through larger clinical studies.
Paving the Way for Personalized Treatment Strategies
Professor Patrick Tan, Senior Vice-Dean for Research at Duke-NUS, said that he sees potential in the new test. He added:“This innovation isn’t just a step forward in cancer therapy, it’s a significant advancement in patient care that could extend across multiple diseases. By offering clinicians real-time data on the functionality of these engineered T cells, we are paving the way for highly personalized treatment strategies that could significantly enhance patient outcomes.”
Reference:
- Duke-NUS scientists develop novel plug-and-play test to evaluate T cell immunotherapy effectiveness - (https://www.duke-nus.edu.sg/newshub/media-releases/media-releases/plug-and-play-test-to-evaluate-t-cell-immunotherapy-effectiveness)
Source-Eurekalert