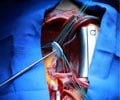
Scientists have developed a new polymer TAVR valve.
‘The TAVR valve was made by placing pellets of the raw polymer into a mold under heat and pressure for one hour using compression molding. ’





The experimental Polynova valve was compared with tests on two valves that are currently in use in patients, the Perimount SAVR used in open heart surgery and the Inovare TAVR used in the minimally-invasive procedure. Both are animal tissue valves. Hydrodynamic tests evaluated how the valves perform as an imitation blood fluid is pumped through them at the same rate as a heartbeat. In the baseline test, fluid was pumped through the valves to observe how well the valves opened. The size of the opening when the fluid is pumped through is called the effective orifice area (EOA). A large EOA as well as a more rounded EOA indicates the best performance. The EOA of the polymer valve was both larger and more rounded than both the SAVR and TAVR tissue valves.
For the second test the valves were mounted in a 3D printed model of the aorta of an actual patient. “Patient-specific models are reconstructed from CT scans of candidates for TAVR, using customized computer algorithms,” said Bluestein. “The reconstructed anatomy is then used to simulate TAVR deployment as well as advanced computational studies of fluid dynamics in this ‘virtual patient.’ We can then identify potential problem areas that inform redesign to optimize valve performance.”
In both tests, changes in fluid pressure as it passed through each valve were measured. For all three valves, performance dropped slightly in the patient-specific test compared to the mechanical device used in the first test. This was expected because the variation in the patient’s anatomy puts various pressures on all the valves resulting in shape changes and a slightly less efficient flow. Nonetheless, there were only slight differences between valves in terms of performance in both test environments, which were insignificant.
The final test measured the activation of platelets as they flow through each valve. Platelets are the tiny vesicles in the blood that cause blood clots to form when activated. The Bluestein group has focused on developing implanted valves and heart pumps that are designed to reduce clotting. These implants can cause perturbations in blood flow that activates the platelets and initiates the clotting cascade, which increases the formation of blood clots and the risk of stroke,
Advertisement
The results of the clotting tests indicated that the polymer valve was the least likely to activate platelets.
Advertisement
The work was published in the January 2019 issue of the Annals of Biomedical Engineering2.
This project was supported by National Institute of Biomedical Imaging and Bioengineering Quantum Award Phase II-U01EB012487, the National Heart Lung and Blood Institute grant STTR R41-HL134418, and the Center for Biotechnology: a New York State Center for Advanced Technology, New York State Department of Economic Development.
Source-Newswise