The targeted drug, palbociclib, was granted accelerated approval by the US Food and Drug Administration for use in women with advanced breast cancer.
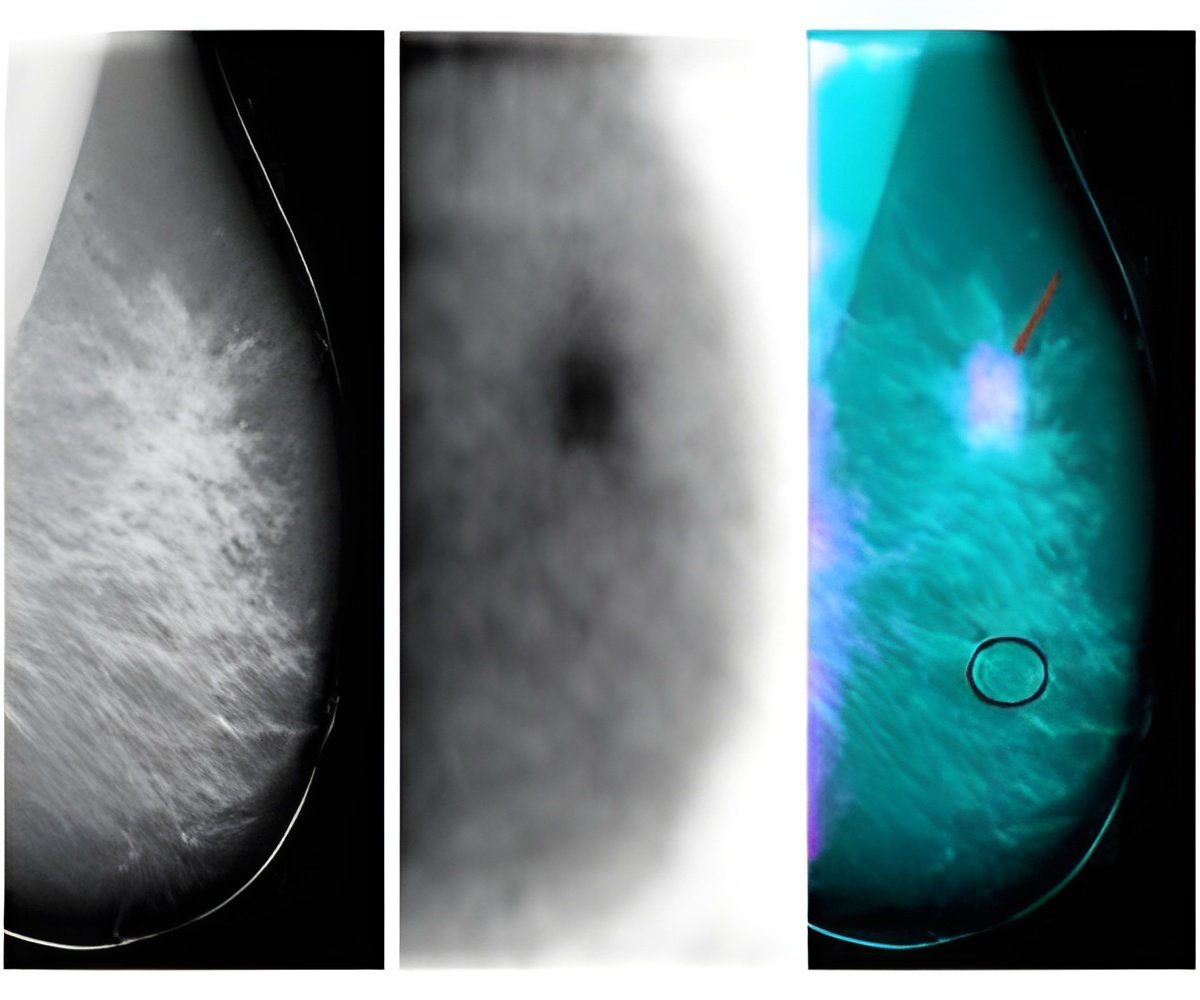
The targeted drug, palbociclib, is made by Pfizer and was granted accelerated approval by the US Food and Drug Administration earlier this year for use in women with the most common form of advanced breast cancer, known as estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-).
According to the findings of a phase III trial presented at the ASCO meeting, the drug -- when used in combination with an anti-estrogen agent called Fulvestrant -- was able to double the time women spent without having their cancer advance.
The combination delayed disease progression for just over nine months, compared to nearly four months in women taking Fulvestrant alone, according to a randomized study of 521 women, most of whom were post-menopausal.
Those results led investigators to stop the trial early because it was so effective.
"After initial hormonal therapy stops working in metastatic breast cancer, the next step is typically chemotherapy, which can be effective, but the side effects are often very difficult for women," said lead study author Nicholas C. Turner, a consultant medical oncologist at The Royal Marsden and a team leader at The Institute of Cancer Research, London, United Kingdom.
Advertisement
Palbociclib works by blocking a key protein that fuels the growth of hormone receptor-positive breast tumors.
Advertisement
- Post-diagnosis survival -
Another study released at ASCO involved more than 3,100 postmenopausal women with a localized form of breast cancer, known as ductal carcinoma in situ (DCIS), which is typically treated by surgically removing the cancerous lump from the breast, followed by radiation.
Women with this kind of breast cancer face a higher risk of invasive breast cancer, but death from DCIS itself is rare.
Doctors typically prescribe drugs that block estrogen for a period of five years after surgery to ward off a return of the cancer, either tamoxifen or another class of drugs known as aromatase inhibitors.
In the first large trial of its kind to compare one of these aromatase inhibitors -- anastrazole -- to tamoxifen, researchers found anastrazole was slightly better.
After 10 years, 93.5 percent of women in the anastrazole group were living breast cancer-free, compared to 89.2 percent in the tamoxifen group.
The 10-year overall survival rates were about the same in the two groups (92.5 percent for anastrazole and 92.1 percent for tamoxifen).
"The good news is tamoxifen and anastrazole are both very effective, but it seems that women have better chances of staying well with anastrazole," said lead study author Richard Margolese, a professor of surgical oncology at The Jewish General Hospital, McGill University in Montreal, Canada.
- Surgery findings -
A third study released at ASCO and published in the New England Journal of Medicine found that women who have a bit of extra tissue removed around the tumor during breast-conserving surgery, or partial mastectomy, face a reduced risk that cancer will be left behind.
The study involved 235 women with breast cancer diagnoses ranging from stage 0 to 3, some of whom were randomly assigned to have extra tissue around the tumor removed -- known as cavity shave margins (CSM) -- and some who were not.
"Despite their best efforts, surgeons could not predict where the cancer was close to the edge," said lead author Anees Chagpar, associate professor of surgery at the Yale School of Medicine.
But those who had cavity shave margins were half as likely to need surgery again, the study found.
Patients will be followed for five years to see if survival or recurrence are influenced by the procedure.
"This randomized controlled trial has the potential to have a huge impact for breast cancer patients," Chagpar said.
"No one likes going back to the operating room, especially not the patients who face the emotional burden of another surgery."
Source-AFP