New approach of mixing two medicines typically associated with malaria and melanoma can treat pancreatic tumors with KRAS mutations, said researchers.
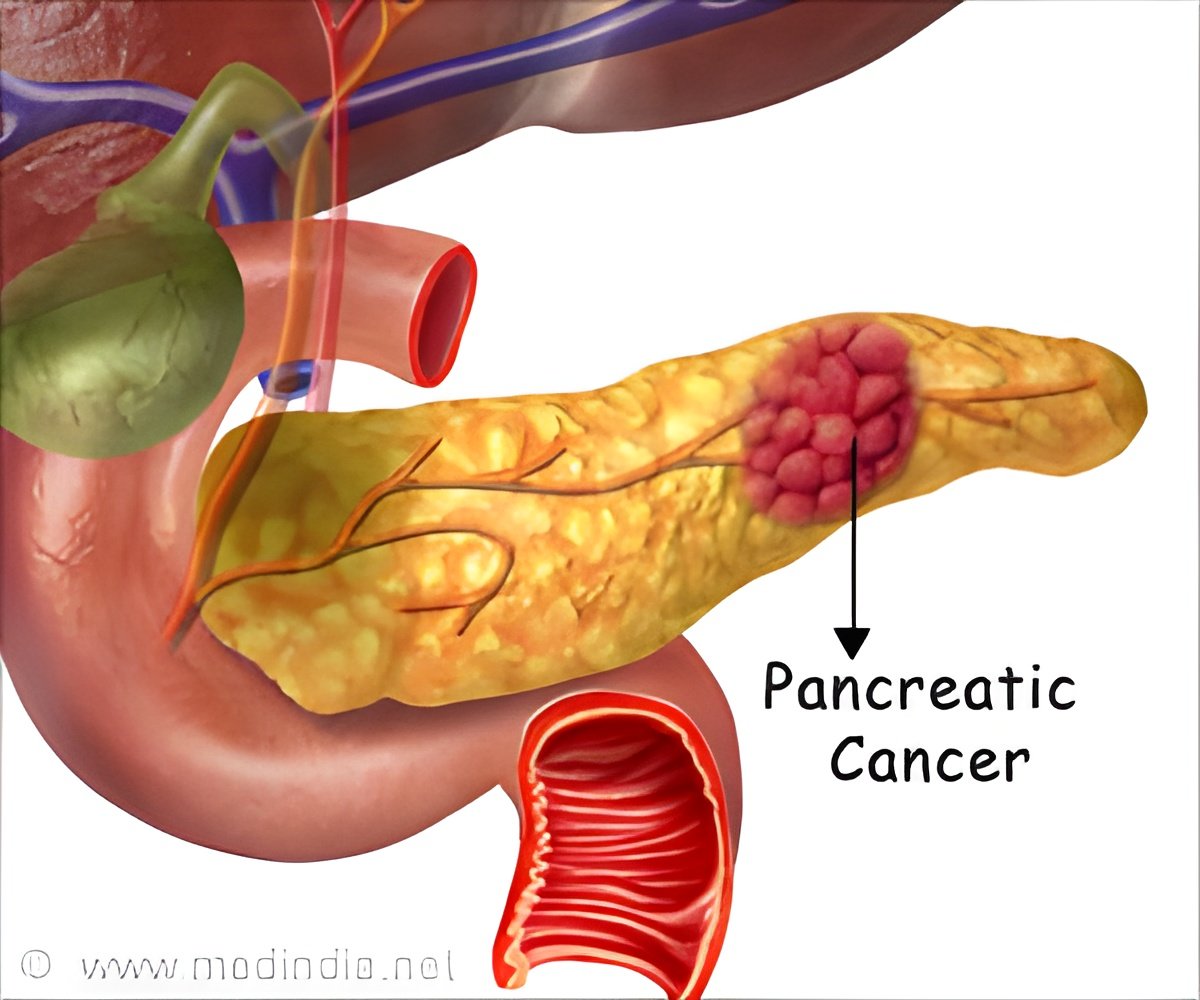
‘Scientists have discovered a combination drug therapy that may effectively combat pancreatic cancer. ’





"In publishing how this new approach of mixing two medicines typically associated with malaria and melanoma can treat pancreatic tumors with KRAS mutations, we are hoping the clinical trial supported by the Pancreatic Cancer Collective's New Therapies Challenge Grant will help us learn how to best administer these medicines, so that we can maximize the benefit for as many patients as possible," said Dr. Tuveson. "Dr. McMahon's research shows promise as an important advance and was selected in the Pancreatic Cancer Collective as part of the "New Therapies Challenge" for support for further validation as therapy. In this Challenge program, a second round of funding, up to $4 million, is possible to support additional clinical studies to bring new treatments to patients faster," added Phillip A. Sharp, PhD, the Nobel laureate who is chair of SU2C Scientific Advisory Committee (SAC) and scientific co-leader of the Collective.
Dr. McMahon's project, "Pancreatic Cancer Collective Research Team: Combined Targeting of MEK1/MEK2 and Autophagy for Pancreatic Cancer" will work toward two goals: 1) elucidating the mechanism(s) by which autophagy is regulated by the RAS pathway, in order to identify predictive biomarkers and new autophagy inhibitors that might be tested in clinical trials; and 2) performing a clinical trial of the T/HCQ combination therapy.
Source-Eurekalert