New treatment for multidrug-resistant tuberculosis has been approved by US health regulators - the first such federal approval aimed at tackling the deadly disease in 40 years.

But the regulator cautioned that the new drug should be used sparingly.
"Sirturo provides much-needed treatment for patients who have don't have other therapeutic options available," announced Edward Cox of the Office of Antimicrobial Products in the FDA's Center for Drug Evaluation and Research.
"However, because the drug also carries some significant risks, doctors should make sure they use it appropriately and only in patients who don't have other treatment options."
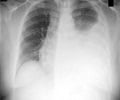
TB is an infection caused by Mycobacterium tuberculosis and is one of the world's deadliest diseases. It is spread from person to person through the air and usually affects the lungs, but it can also affect other parts of the body such as the brain and kidneys.
Nearly nine million people around the world had TB in 2011, including more than 400,000 suffering from a multidrug resistant form of the disease, according to the World Health Organization.
Advertisement
The warning also noted deaths in patients treated with Sirturo and that nine patients who received Sirturo died -- compared with two patients who received a placebo.
Advertisement
Source-AFP