After-effects of infected grafts may result in infections, a new way to enhance detection and treatment of vascular graft infections is developed.
The study represents the after-effects of infected grafts, which includes the formation of biofilms that can house bacteria and serve as a source of recurrent infection. This may enable to develop better strategies for diagnosing and managing vascular graft infections.
"Staphylococcus aureus (S. aureus) is one of the leading causes of infected grafts because it readily adheres to the surface of the implanted device and forms thick biofilm layers. Biofilms can shelter bacteria from the patient's immune responses or antibiotic treatment. These biofilm layers are difficult to detect because they are often unaccompanied by clinical symptoms," noted lead investigator Bettina Löffler, MD, Director of the Institute of Medical Microbiology, Jena University Hospital (Germany). "Currently, there are no effective treatment strategies against these infections. Biofilms require antibiotic concentrations up to 1000 times higher than normal and these concentrations are not clinically feasible. It is of great importance to understand the underlying pathogenesis of biofilm formation on vascular grafts in order to find quick and effective treatment possibilities without having to resort to invasive procedures such as surgical removal."
The researchers developed a new mouse model that more closely mimics the human condition. The catheter is placed within a blood vessel (the right carotid artery) and bacteria reach the catheter via the blood stream (bacteria are introduced into tail veins seven days after the catheter is inserted). "Just as in humans, with this model the bacteria need to overcome the stress of the blood flow, the shear stress induced by the blood flow, and the host's immune system to form a biofilm infection on the catheter," explained Dr. Löffler. By establishing this novel model in mice investigators opened up the possibility to use the vast array of genetically manipulated mice available, which will allow the study of many different aspects of the disease and identification of better and more reliable treatment and detection strategies for vascular graft infections.
An interesting finding of the study was that all S. aureus strains tested formed biofilms in vivo, regardless of whether they formed high biofilm levels in cell culture. This finding demonstrates that colonization of vascular grafts in vivo is a general characteristic of all S. aureus infections and that these bacteria are highly adaptive to their environment.
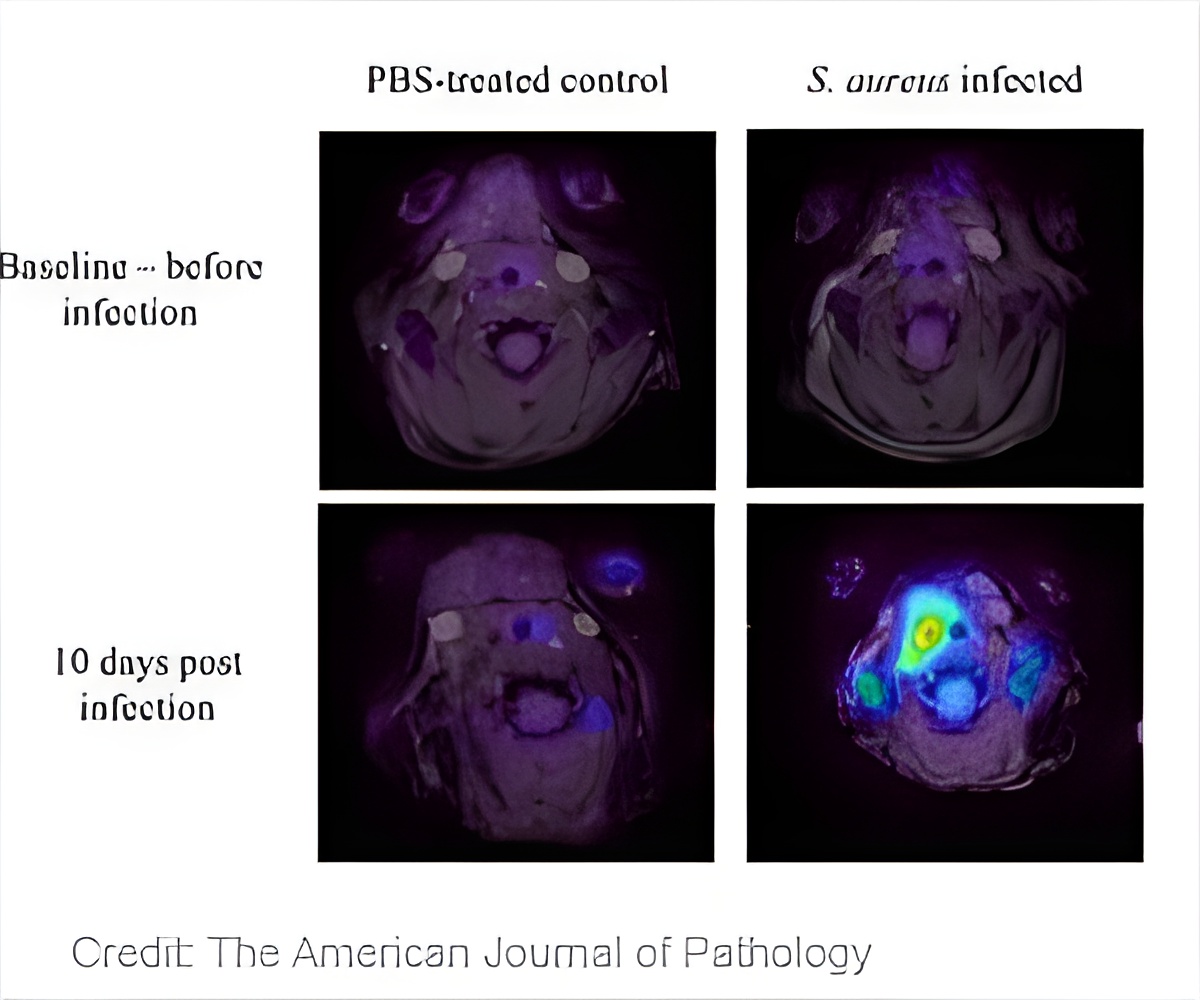
Using PET imaging, the investigators discovered a high level of inflammation at the site of the catheter during vascular graft infections. MR imaging revealed that blood flow velocity was decreased through the catheter due to infection and biofilm formation.
Advertisement
Advertisement