A novel way to block the action of genetic mutations found in nearly 30 percent of all cancers has been discovered by University of Illinois researchers.
‘Development of effective renin-angiotensin system (RAS) inhibitors represents a 'holy grail' in cancer biology.’





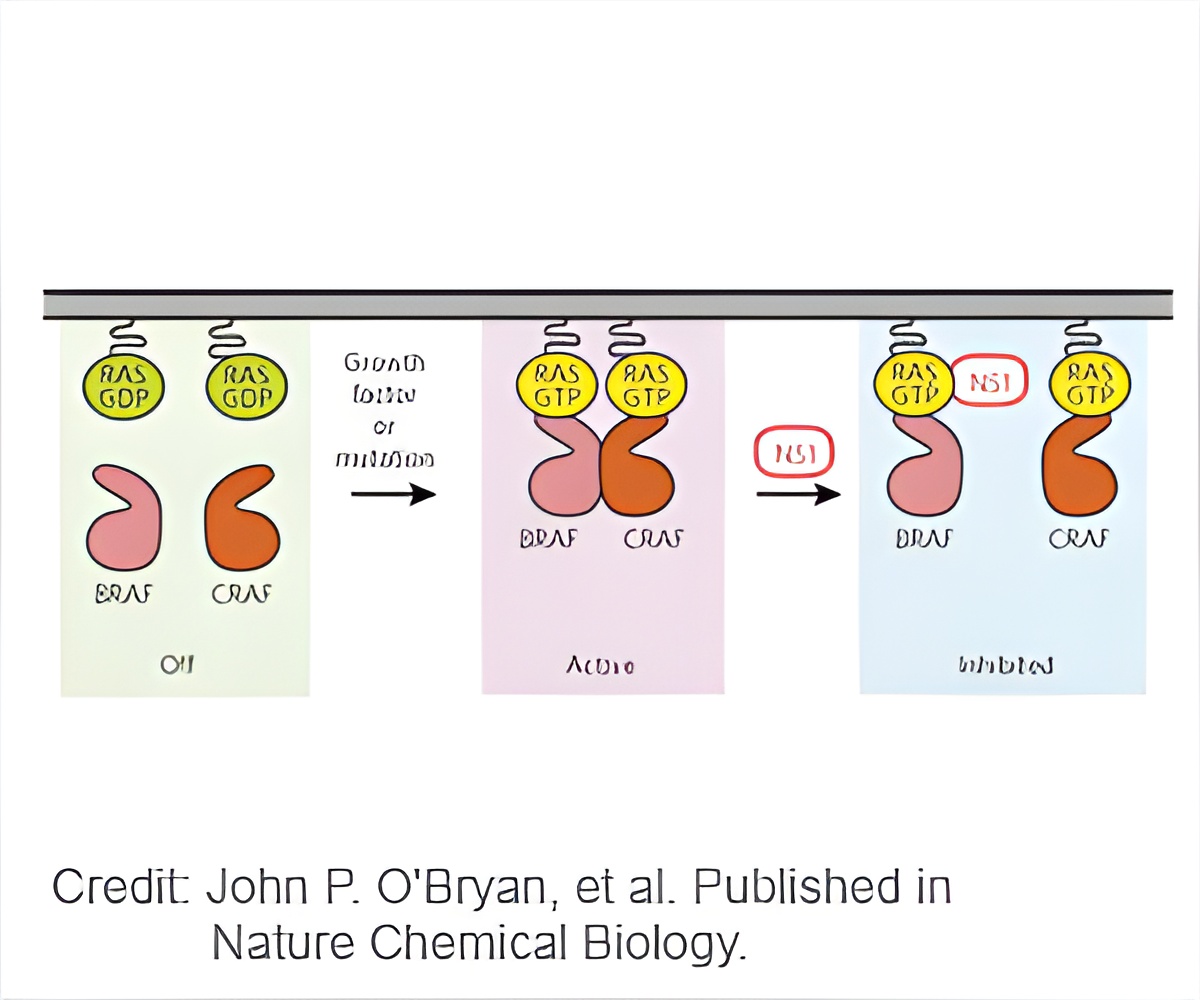
The group of proteins include three members, K-RAS, H-RAS and N-RAS. The prevalence of RAS mutations in human cancers and the dependence of tumors on RAS for survival has made a RAS a prime target for cancer research and drug discovery. Scientists and drug developers have long studied RAS oncogenes hoping to find a new treatment for cancer, but they have not yet been able to identify drugs that safely inhibit the oncogene's activity.
John O'Bryan, associate professor of pharmacology in the UIC College of Medicine, led a team of researchers that took a different approach to studying RAS, and discovered that a synthetic binding protein they call "NS1 monobody," which they created in the lab, can block the activity of the RAS proteins.
"We did not look for a drug or specifically for an inhibitor," said O'Bryan, who is also a member of the University of Illinois Cancer Center and holds an appointment at the Jesse Brown VA Medical Center in Chicago. "We used monobody technology, a type of protein-engineering technology, to identify regions of RAS that are critical for its function."
Unlike conventional antibodies, monobodies are not dependent on their environment and can be readily used as genetically encoded inhibitors, O'Bryan said.
Advertisement
Monobodies were developed by Shohei Koide, a co-author on the study who is professor of biochemistry and molecular pharmacology at New York University. They have been used to target a diverse array of proteins that include enzymes and receptors.
Advertisement
O'Bryan says the findings, published in the journal Nature Chemical Biology, provide important insight into long-standing questions about how RAS proteins function in cells. These insights may help guide the development of new therapeutic approaches to treating cancer by interfering with mutant RAS function in cancer cells.
"We now have a powerful tool we can use to further probe RAS function. While future studies and trials are needed before these findings can be leveraged outside the lab, this study provides new insight into how we can potentially inhibit RAS to slow tumor growth."
Source-Eurekalert