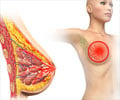
The Government's health watchdog has given the final draft of approval for the breakthrough drug for the treatment of breast cancer.
A new class of breast cancer drugs had yesterday won the final draft of approval for use in the NHS alongside the ‘gold standard’ Tamoxifen.
The National Institute for Health and Clinical Excellence (Nice) had issued a final draft on the appraisal for the use of three types of aromatase inhibitors in postmenopausal women with early breast cancer. It was explained that these drugs would act by reducing the risk of tumors spreading after surgery and might benefit thousands of women with hormone receptor positive early breast cancer.It was reported that the three drugs were Arimidex (anastrozole), Femara (letrozole), and Aromasin (exemestane) and will be available on the NHS alongside the "gold standard" drug Tamoxifen. It was also explained that though there is still the probability of the final guidance subjected to an appeal, it would be highly unlikely to be stopped at this stage. The inhibitors act by stopping the natural production of oestrogen, which is the hormone that is responsible for the growth and recurrence of many breast cancers.
Reports have indicated that a study conducted in, co-ordinated by Cancer Research UK, had found that patients who switched from Tamoxifen to Aromasin halfway through treatment had reduced the risk of the disease recurring by a third. It was also stated that in June, the landmark Intergroup Exemestane Study had also found that switching over to Aromasin had cut the risk of death by 17%, when compared with those who remained on Tamoxifen.
A trial with Arimidex that was given immediately after surgery had showed an extra 26% reduction in recurrence of cancer further to the 50% reduction provided by Tamoxifen. Studies have also shown that Femara is also more effective than Tamoxifen in a number of studies. The government had already announced that the doctors could use the drugs as they see fit, without waiting for recommendation from NICE.