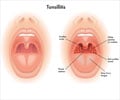
A study found no link between corticsteroid use in children during a tonsillectomy and serious bleeding events following surgery.

"A recent randomized trial studying the dose response of perioperative dexamethasone to postoperative nausea and vomiting [PONV] in children undergoing tonsillectomy was prematurely terminated due to an increased risk of postoperative hemorrhage. The outcomes of the trial suggested that a single dose of intraoperative dexamethasone significantly increased post-tonsillectomy hemorrhage events. In light of these findings, there is a need to reassess the safety profile for dexamethasone when used during tonsillectomy," the authors write.
Thomas Q. Gallagher, M.C., U.S.N., of the Naval Medical Center Portsmouth, Va., and colleagues performed a clinical trial to examine bleeding events in children associated with dexamethasone use during tonsillectomy. The multicenter, randomized trial included 314 children ages 3 to 18 years undergoing tonsillectomy without a history of bleeding disorder or recent corticosteroid medication use. The study was conducted between July 2010 and December 2011 with 14-day follow-up. The researchers tested the hypothesis that dexamethasone would not result in 5 percent more bleeding events than placebo using a noninferiority (outcome not worse than treatment compared to) statistical design. Patients received a single perioperative dose of dexamethasone or an equivalent volume of saline.
The primary outcome measured was rate and severity of post-tonsillectomy hemorrhage in the 14-day postoperative period using a bleeding severity scale (level I, self-reported or parent-reported postoperative bleeding; level II, required inpatient admission for postoperative bleeding; or level III, required reoperation to control postoperative bleeding).
One hundred fifty-seven children (median [midpoint] age, 6 years) were randomized into each study group, with 17 patients (10.8 percent) in the dexamethasone group and 13 patients (8.2 percent) in the placebo group reporting bleeding events. The overall rate of bleeding events for all levels was 30 out of 314 (9.6 percent). The researchers found that in an intention-to-treat analysis, the rates of level I bleeding were 7.0 percent (n = 11) in the dexamethasone group and 4.5 percent (n = 7) in the placebo group; rates of level II bleeding were 1.9 percent (n = 3) and 3.2 percent (n = 5), respectively; and rates of level III bleeding were 1.9 percent (n = 3) and 0.6 percent (n = 1), respectively.
"The dexamethasone treatment failed to show noninferiority for the level I bleeding, but did demonstrate that the bleeding rate with dexamethasone is not more than 5 percent greater than that with placebo (noninferiority) for both level II and III bleeding events. The data was stratified for primary vs. secondary bleeding events and a decrease in level II and level III bleeding events in both groups was noted," the authors write.
Advertisement
Advertisement