The precise 3-D atomic structure of a thin protein filament critical for cells in the inner ear is being mapped by researchers and they calculated the force necessary to pull it apart.
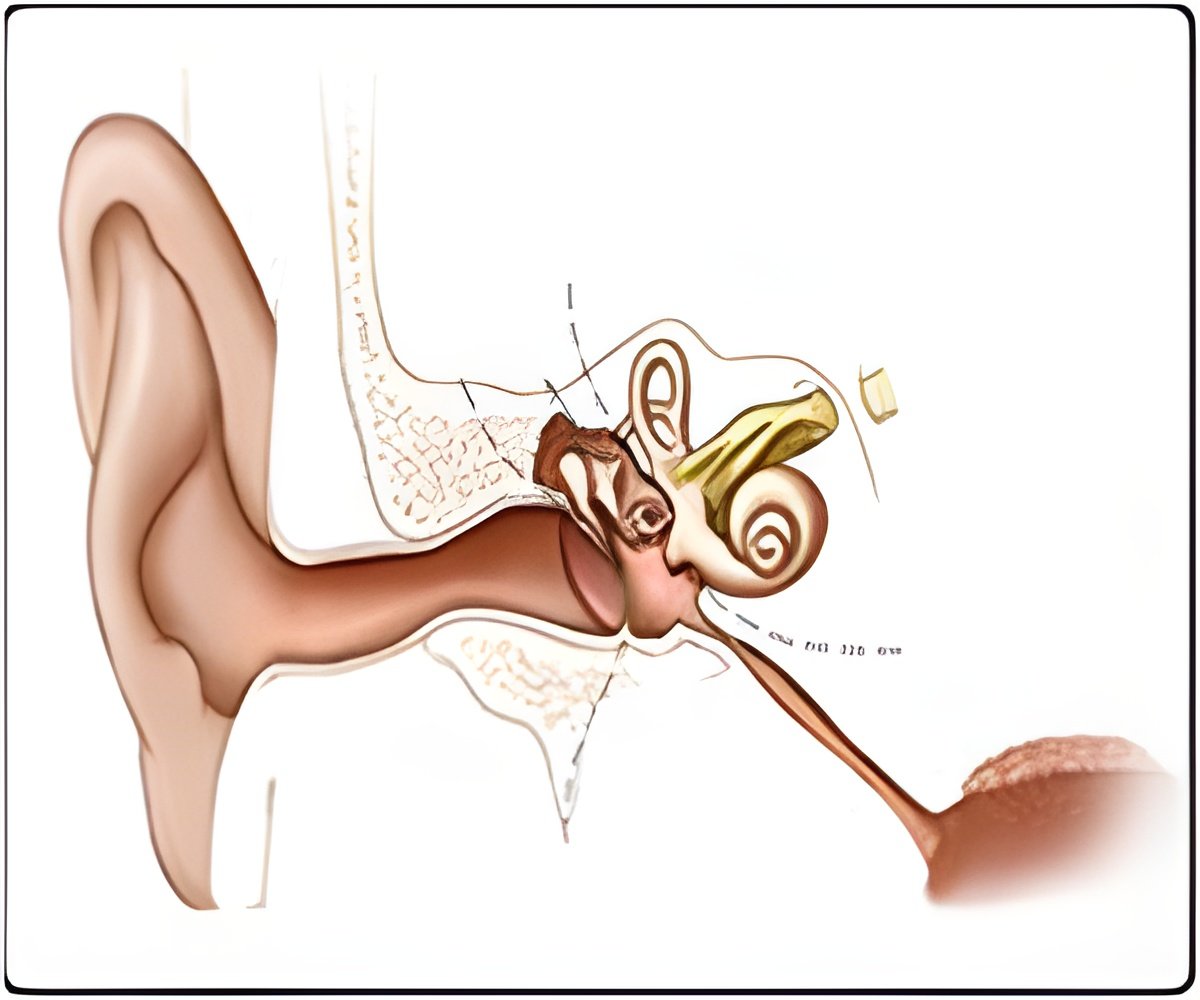
"Tip links are absolutely vital to hair cells, and hair cells are absolutely vital for hearing and balance," said Corey. "We now have this new understanding of how noise can break a tip link and potentially cause a hearing problem."
The sensations of hearing and balance rely on hair cells, a family of cells located in the inner ear. Crucial to their function are tip links, strings of protein that physically connect the cilia or "hairs" found on these cells. When the cilia move in response to sensory stimuli — head movement or the vibration of sound, for example—tension is applied to the tip links, which begins a process that ultimately sends nerve impulses to the brain.
Tip links are composed of two different types of proteins called cadherins, which connect in the middle to make one long string. Mutations in these proteins often result in congenital deafness and balance disorders. Scientists have only recently made strides toward understanding the nature of these cadherins, especially at their connection to each other—hypothesized to be the first area to break under stress.
To test this, Marcos Sotomayor, first author on the paper and a postdoctoral researcher in the lab of David Corey, investigated the structure of that bond.
He first synthesized and purified a large amount of the very ends of the proteins, where they connect. After crystallizing the purified proteins bound to each other, the team took advantage of powerful X-rays generated by a 3000-foot long electron accelerator at Argonne National Laboratory.
Advertisement
Sotomayor analyzed the diffraction pattern generated at Argonne and coupled it with biochemical data. From this, he created a complete 3-D map of the structure of bonded region, down to the position of each individual atom.
Advertisement
With this precise 3-D structure, the team used supercomputers to simulate the dynamics of these proteins under a variety of conditions, including applying forces to pull the bond apart. They found that it takes only half as much force to break the connection as it does to unfold the cadherins themselves—confirming that the bond is indeed the weakest region of the tip link. They suggest the tip link is strong enough to withstand normal sound, but the connection is likely the first to break under loud noises.
They also showed how certain mutations that produce deafness could weaken the bond, perhaps allowing it to come apart even with quiet sound.
The group plans to characterize tip-link structure further, moving on to other parts such as where the cadherins link to tension-activated ion channels at the tips of the hair cells' cilia.
Source-Eurekalert










