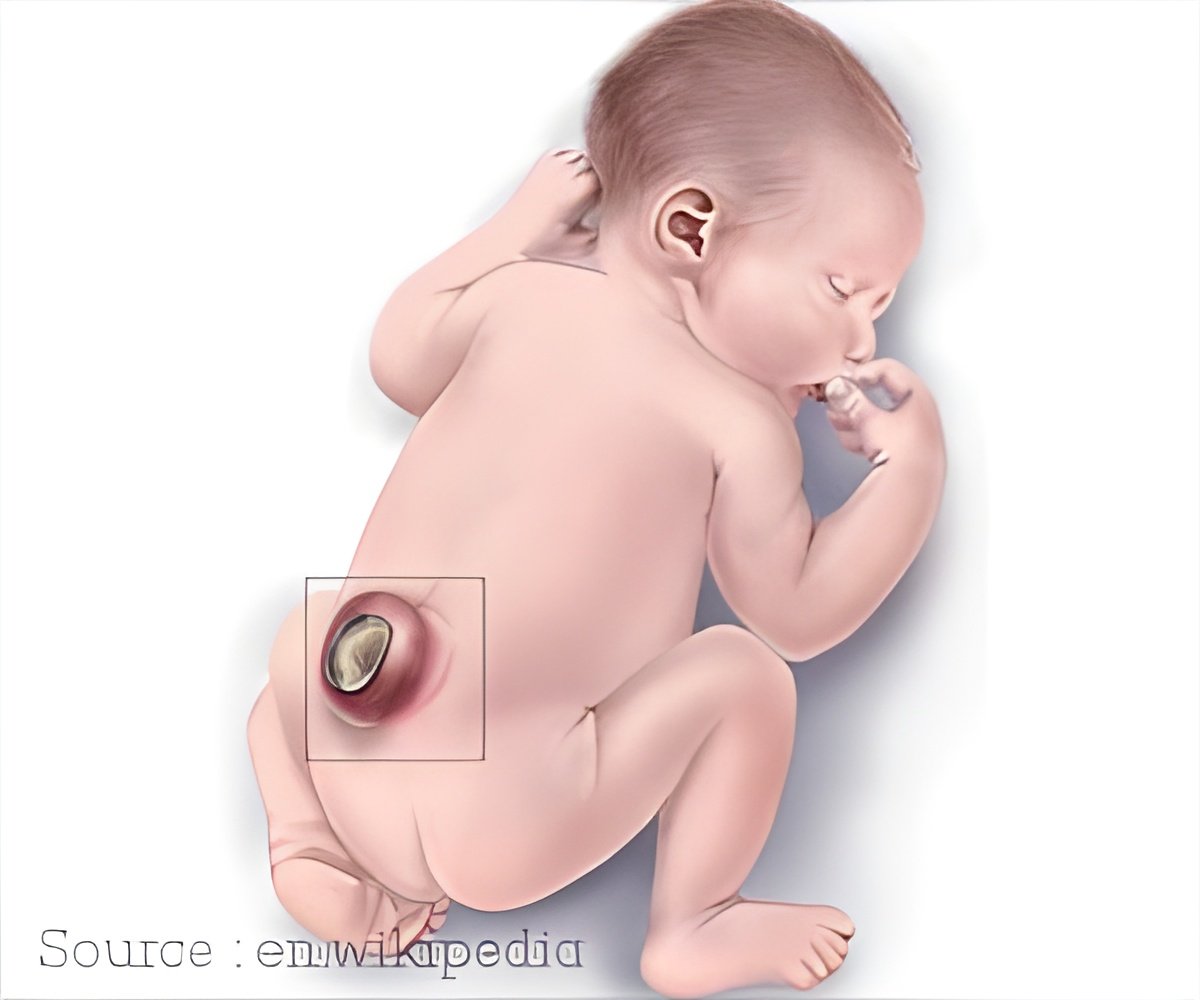
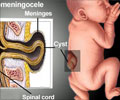
Bioengineering an alternative to open fetal repair for spina bifida. The in utero application of the biomaterial was technically feasible and did not harm fetuses.
TOP INSIGHT
The potential to use the reverse thermal gel in a minimally invasive approach at an earlier gestational age offers a promising alternative to the current in utero repairs for spina bifida.
The recently published research, led by Dr. Marwan and his team, studied the effects of the chemically synthesized, novel RTG called PSHU-PNIPAAm and characterized its ultrastructure by scanning electron microscopy, its stability in amniotic fluids and its permeability.
"Our team’s specific aim was to study the effects of the chemically synthesized material on the basic cellular functions of mouse neonatal fibroblasts, keratinocytes and neurons - the types of cells exposed in open NTDs, and inject the material into mouse embryos in utero," said Dr. Marwan, who is also an assistant professor of surgery at the CU School of Medicine.
"We found that the in utero application of the biomaterial was technically feasible and did not harm fetuses. The RTG successfully formed a gel and attached to the skin, demonstrating successful in utero suitability as a potential alternative for NTD closure."
As studies continue, Dr. Marwan and his team will explore the ability of the RTG to act as a scaffold for cellular interaction, and they will make additional chemical modifications to incorporate biomolecules that mimic the extracellular matrix environment of a fetus and enhance cellular activities.
Source-Eurekalert
 MEDINDIA
MEDINDIA

 Email
Email






