Bioresorbable polymer-based drug-eluting stents (BP-EES) are comparable to durable polymer-based drug-eluting stents (DP-ZES).

‘High rate of strut coverage and low rates of neoatherosclerosis with thin strut bioresorbable polymer-based and durable polymer-based drug-eluting stents has been observed in imaging studies.’





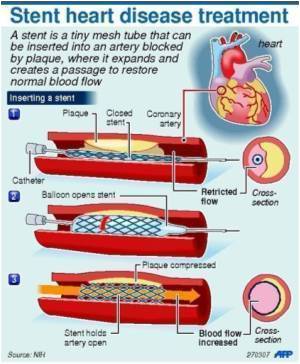
To mitigate the risk related to durable polymer (DP), bioabsorbable
polymers (BP) that coat only the abluminal side of the stent, avoiding
contact with circulating blood, and render stent surface similar to
those of BMS after complete absorption, have been developed. Whether BP
abluminal coating may counteract in-vivo delayed healing and NA
development in current thin-strut DES is unknown. TRANSFORM-OCT randomized 90 patients with multivessel disease (1:1) to a BP everolimus-eluting stent (BP-EES, Synergy) or a DP zotarolimus-eluting stent (DP-ZES, Resolute Integrity). The primary endpoints were maximum length of consecutive frames with uncovered struts at three months (powered for non-inferiority of BP-EES) and the percentage of patients presenting with frames of NA at 18 months (powered for superiority of BP-EES) as measured by OCT.
The three-month median percentage of covered struts was 79.1 (IQR 60.4, 89.8) for BP-EES and 78.4 (IQR 62.1, 87.8) for DP-ZES, P=0.93. The 18-month median percentage of covered struts was 99.4 (IQR 96.6-100) for BP-EES and 98.0 (IQR 94.4-99.8) for DP-ZES, P=0.14. The co-primary endpoint of in-stent NA at 18 months from 98.9% of all eligible patients was 11.6 % for BP-EES versus 15.9% in DP-ZES (P=0.59) with low percentage of frames with NA in both stent types (1.1 ± 3.1 for BP-EES versus 2.5 ± 9.1 for DP-ZES, P=0.33)
"In this head-to-head in-vivo comparison of early and late healing response, the bioresorbable abluminal polymer Synergy everolimus-eluting stent was non-inferior at three-month and similar at 18-month follow-up to the durable conformal polymer Resolute zotarolimus-eluting stent," said Giulio Guagliumi with Ospedale Giovanni XXIII in Bergamo, Italy. "TRANSFORM-OCT adds a novel mechanistic dimension to the assessment of new-generation drug-eluting stents, consolidating the understanding that well designed and biocompatible polymers, regardless of whether they are durable or biodegradable, may favorably impact the long-term vascular response of these stents."
Source-Eurekalert