Novel drug holds promise for treatment of Rheumatoid Arthritis
The successful completion of Phase II clinical trials of a novel drug called dnaJP1 for treatment of osteoarthritis that works without suppressing the immune system has been announced by Researchers at the University of California.
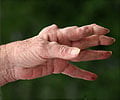
Rheumatoid arthritis is a chronic, painful disease affecting millions worldwide. It is thrice more common in women in men and affects people of any age. It strikes joints on both sides of the body, such as the hands or feet or knees but can also affect the skin, eyes, lungs, heart, blood, nerves or kidneys. It is an incurable disease, with most therapies focusing on symptom relief.Current medications for treating RA range from anti-inflammatory drugs, such as aspirin, to corticosteroids and medicines that alleviate symptoms by suppressing or killing the body’s immune response, basically crippling the body’s ability to defend itself against other infectious diseases or cancer.
The novel drug is a peptide derived from a naturally occurring protein, dnaJ, which generates inflammation in RA patients. The drug when administered disturbs the immune system, which is then activated to kill and clear foreign pathogens in the body. This results in an enhanced immune response which is not possible otherwise. The drug would have to administered through the oral route as the immune set up in the gut has the potential to educate the body to view a protein as one that isn’t dangerous or foreign. DnaJP1 was found effective in a double-blind, placebo-controlled trial sponsored by the National Institutes of Health, which took place between 2000 and 2005 and involved about 160 patients.
Patients received 25mg of dnaJP1 daily by mouth for six months, and the treatment was found to be safe and well-tolerated. When compared with a placebo, patients in the treatment group experienced lessening of symptoms such as swollen joints, tenderness, pain and decreased mobility. Improvement was particularly significant at the follow up visits, indicating a lasting effect of the drug.
The first two trials of dnaJP1 have not raised any significant safety concerns and offer an improved treatment option for patients with rheumatoid arthritis, especially while taking into consideration the side effects associated with current available therapy. The researchers intend to get approval and funding to move into Phase III clinical trials.
This is a very exciting and novel therapeutic approach, which holds the promise to be an entirely new type of immunomodulatory drug – one that can shape a patient’s immune system, rather than suppressing it. With such an advance, the treatment of rheumatoid arthritis seems a potential reachable reality.