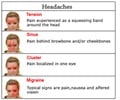
Patients with severe headaches were treated with TOPOFEN gel. They were three times as likely to be completely relieved from nausea and sound hypersensitivity.

The study was conducted by scientists from Achelios and the Michigan Headache and Neurological Institute in Ann Arbor, Michigan. The randomized placebo-controlled study involved 48 adults with a history of episodic migraine. Patients were instructed to treat five moderate to severe migraines by applying the TOPOFEN gel on the skin over the three branches of the trigeminal nerve.
The trigeminal nerve is a nerve responsible for sensation in the face and motor functions such as biting and chewing.
Compared with placebo, TOPOFEN resulted in greater improvement in pain assessments, a faster time to pain response, a reduction of migraine-associated symptoms such as nausea, light and sound hypersensitivity and greater suppression of pain over the 24-hour period that patients were asked to follow each migraine once they applied the gel.
"Of the severe migraine patients, 77% experienced relief of pain and migraine-associated symptoms and 45% had sustained pain relief from two to 24 hours compared to 15% of placebo," the authors noted.
Also 50% of patients, who treated their severe pain with TOPOFEN were pain free in 24 hours compared to 25% of placebo-treated patients.
Advertisement
Some patients experienced application-site irritation, predominantly mild or moderate. That was the only reported side effect which resolved quickly.
Advertisement
Achelios Therapeutics is set to announce the results at the American Academy of Neurology’s annual meeting in Washington on April 22.
Source-IANS