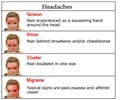
The FDA has approved NuPathe’s battery-powered sumatriptan patch to treat nausea and headaches caused by migraines in adults.

This is NuPathe’s second application for transdermal sumatriptan patch after the FDA had rejected a similar product, known as Zelrix, back in August 2011, citing concerns over manufacturing and patient’s safety.
NuPathe’s CEO Armando Anido said that the company expects to bring the product in the market by the end of this year. “We anticipate the product will be available for sale in the fourth quarter of this year. We’re right now in conversations with a number of people for partnership in the United States”, Anido said.
Source-Medindia