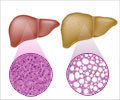
Primary biliary cholangitis is a chronic liver disease where the bile ducts in the liver become inflamed causing accumulation of bile, leading to cirrhosis.
‘The U.S. Food and Drug Administration granted accelerated approval for Ocaliva (obeticholic acid) for the treatment of primary biliary cholangitis (PBC).’





The only approved treatment for PBC was ursodeoxycholic acid (UDCA) and patients who respond inadequately to this treatment can use Ocaliva in combination with UDCA.Ocaliva interacts with farnesoid X receptor (FXR), which plays a key role in bile acid regulation. By binding to this receptor Oclavia suppresses bile accumulation and increases the flow, leading to a reduction in bile levels in the liver.
The drug was tested in 216 participants, wherein after one-year of follow-up, reductions in bile levels was higher among Ocaliva-treated participants compared to placebo-treated participants.
Some of the side effects of Ocaliva include fatigue, abdominal pain, throat pain, dizziness and itching of the skin. Ocaliva is manufactured by New York-based Intercept Pharmaceuticals.
Source-Medindia