The mechanism behind oncogene addiction in acute leukemia has been revealed by CSHL scientists.

"The crucial questions are what MLL-AF9 enables cancer cells to do, and how we might intervene to suppress these effects – in other words, how to use our knowledge of the mechanism behind the cancer cell's addiction for developing highly specific anti-cancer therapies.," says CSHL Adjunct Professor and HHMI Investigator Scott W. Lowe, who directed the research, which is reported in a paper that appears August 1 in the journal Genes & Development. "Until now, we've not been able to answer these questions in living animals."
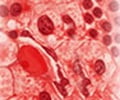
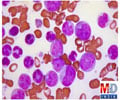
The team's research demonstrated that MLL-AF9 enforces a cellular program that enables blood cells to keep renewing themselves, rather than progressing through the usual stages of cellular life, and eventually dying. While this property of aberrant self-renewal has been a known characteristic of leukemia cells for many years, Lowe and his CSHL colleagues were able to decipher the underlying genetic program in unprecedented detail. The central player orchestrating these cancer-specific self-renewal programs was found to be Myb, a protein known to regulate normal blood cell production that previously also has been implicated in certain subtypes of leukemia.
"MLL-AF9 apparently 'hijacks' Myb to enforce a program of aberrant self-renewal," explains Amy Rappaport, who was a co-first author on the paper, with Johannes Zuber. The team also included Christopher Vakoc, a CSHL Fellow, among others. "The consequences of inhibiting Myb in established leukemia were striking," says Zuber. "Following Myb suppression, mouse leukemia cells invariably lost their aberrant self-renewal ability, resumed their normal cell fate, maturing into white blood cells, and eventually got eliminated." As a consequence, mice harboring this aggressive and chemotherapy-resistant form of AML were cured by inhibiting Myb. The protein's suppression had no adverse impact upon normal white blood cells.
To identify and study genes that are specifically required in cancer cells, the researchers took a systematic approach that demonstrates the power of a series of technological advances made by Lowe's group in concert with several other groups at CSHL. These advances play an important role in CSHL's new Cancer Therapeutic Initiative. The Initiative aims to rapidly identify new therapeutic targets and validate them in mouse models specific for genetic subtypes of human cancer.
In the paper published today, Lowe's team first implemented a rapid strategy to introduce common human AML mutations in mice so that they closely mimicked human AML, both in terms of symptoms and response to treatment. These genetically reprogrammed mice are called "mosaic mice." As a next step, the team used a genetic switch to inactivate the oncogene that gives rise to the "addictive" MLL-AF9 oncoprotein. When MLL-AF9 was suppressed in living mice, the cancers shrank and were soon eliminated altogether from all affected organs. This result confirmed the cancer cells' addiction to MLL-AF9, and provided a unique system to reveal the underlying genetic networks.
Advertisement
The RNA interference technique used in the experiments to block Myb is not applicable in humans, for a variety of still-daunting technical reasons. The hope is to find a small molecule that specifically targets the Myb protein, which can be the basis for a human drug. None has yet been developed, although the search is on.
Advertisement
Source-Eurekalert