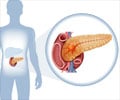
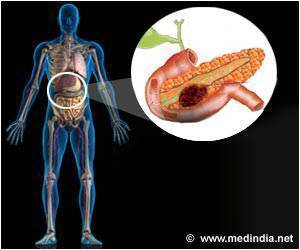
The U.S. Food and Drug Administration has approved Onivyde in combination with fluorouracil and leucovorin to treat patients with advanced pancreatic cancer.
The drug regulator granted Priority Review and orphan drug designations for Onivyde. A study demonstrated the effectiveness of Onivyde. The three-arm, randomized, open label study involved as many as 417 patients with metastatic pancreatic adenocarcinoma whose cancer had grown after receiving the chemotherapeutic drug gemcitabine or a gemcitabine-based therapy.
Investigators found that patients treated with Onivyde plus fluorouracil/leucovorin lived an average of 6.1 months, compared to 4.2 months for those treated with only fluorouracil/leucovorin.
The safety of Onivyde was also tested in 398 patients who received either Onivyde with fluorouracil/leucovorin, Onivyde alone or fluorouracil/leucovorin. Merrimack Pharmaceuticals of Cambridge, Massachusetts holds the marketing authority of Onivyde.
Source-Medindia