Biogen Idec Biotech India, has received approval for the first oral therapy drug for multiple sclerosis, Tecfidera by the DCGI.
Biogen Idec India Acting Managing Director Pooja Vatsyayan said, "We believe Tecfidera provides a new option for patients in India, particularly as it provides the convenience of oral dosing."
The clinical trials conducted for the approval of the drug involved two-phase III studies more than 2600 patients all around the world. Patients from India also were part of clinical research, Biogen Idec India said.
Tecfidera is the first oral drug for the treatment of relapsing-remitting multiple sclerosis (RRMS) in India, it added.
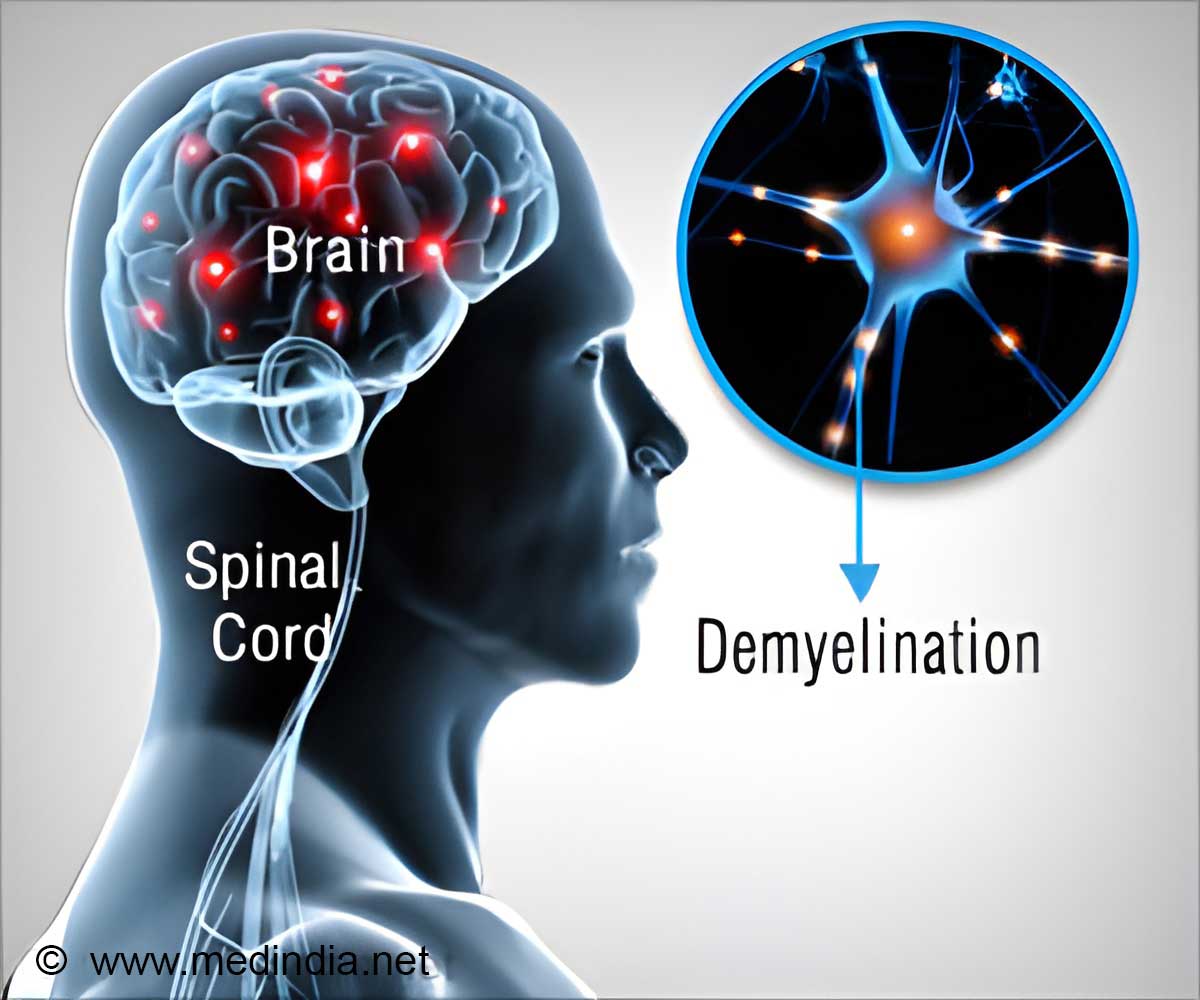
Multiple sclerosis is a disease, which affects brain and spinal cord and is the most common cause of non-traumatic neurological disability afflicting young adults, Biogen Idec India said.
Source-Medindia