By overdosing the cancer cells with oxidative stress can help destroy them, finds a new study.
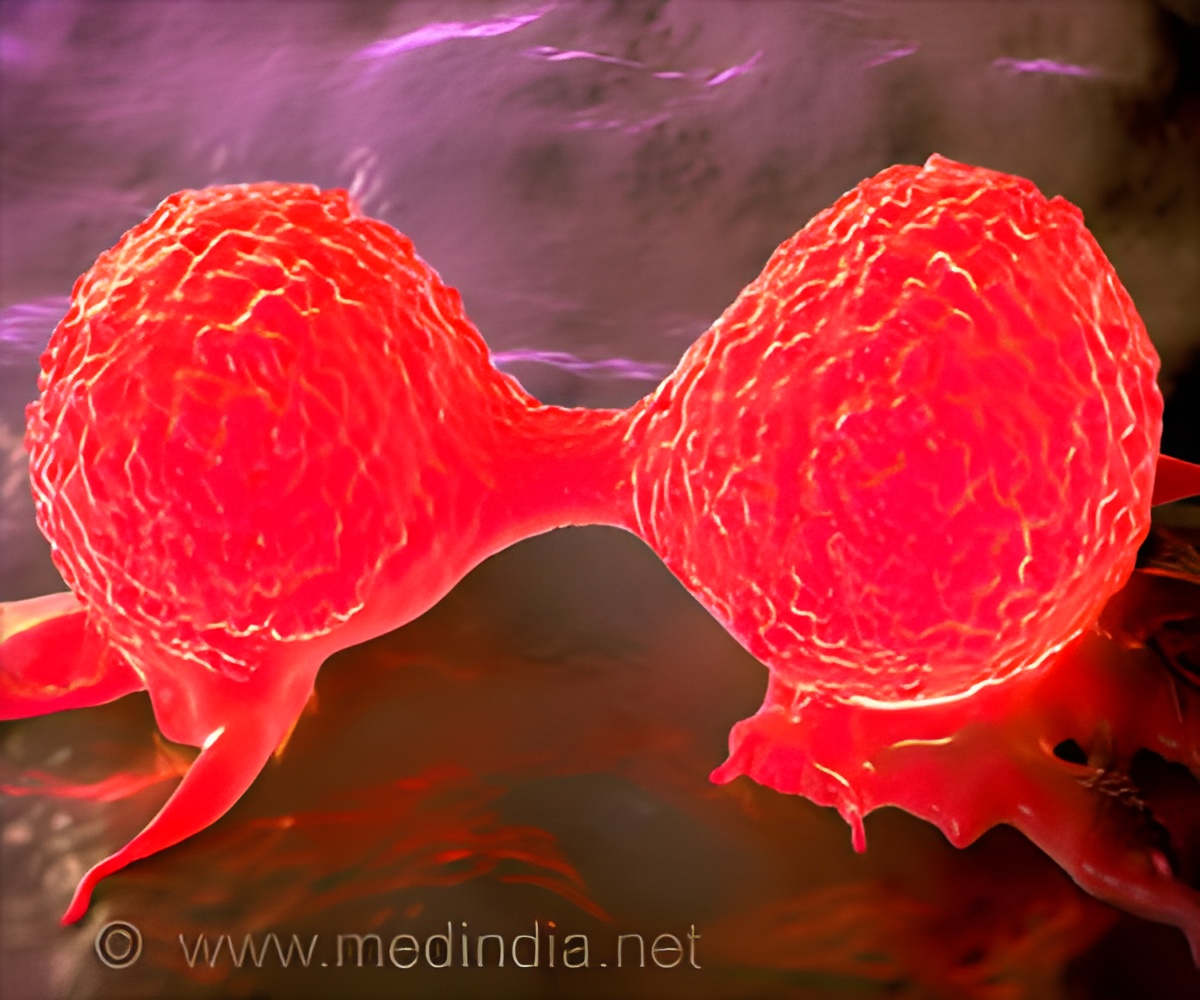
‘Adoptive T cell therapy has been found to reprogram the metabolism of tumor cells, increasing their level of reactive oxygen species, or ROS, leading to their destruction. It is still under development for the treatment of colorectal cancer. This therapeutic approach was already known to poke holes in cancer cells to kill them essentially.’





Scientists treated mice that had large, localized colorectal tumors with adoptive T cell therapy after preconditioning them with a chemotherapy drug known to help with the expansion and persistence of these infused T cells. The T cells are a patient's own cells but engineered to better fight cancer.The therapy appeared to deliver a deadly double-whammy to the cancer cells, says Zhou, corresponding author of the study in the journal Cell Metabolism.
The scientists found the treatment interfered with the production of glutathione, a natural antioxidant found in all cells, as it heightened production and accumulation of ROS inside tumor cells.
Results included increased production by T cells of proinflammatory cytokines - including tumor necrosis factor alpha - which regulate many functions of cancer needs to control like cell proliferation, differentiation, and death.
"We started by asking questions about how immunotherapy can change the metabolism of tumor cells. Our studies show tumor necrosis factor alpha can act directly on tumor cells and induce ROS inside them," Zhou says.
Advertisement
The scientists found similar effects - higher ROS levels correlated with high tumor cell death - when the therapy was used in models of breast cancer and lymphoma.
Scavenging ROS had a similar effect. When they gave the antioxidant N-acetylcysteine - a precursor to glutathione - it also hampered the curative effect of adoptive T cell therapy, they report.
They also found that tumor necrosis factor alpha synergizes with chemotherapy to increase oxidative stress and cancer cell death. And, that giving pro-oxidants - drugs are known to raise ROS levels - can somewhat replicate the tumor-killing benefit of adoptive T cell therapy. It's known that these drugs may increase oxidative stress in cancer cells and push them toward death, or apoptosis, Zhou says.
"Their baseline is already high, and if you further disrupt their ability to deal with these free radicals, they will go toward apoptosis," Zhou says.
In fact, in an apparent failed attempt to fight off the higher ROS, the scientists found increased expression of several antioxidant genes in treated tumor cells.
The significant, cancer-lethal ROS increases they found were limited to the tumor cells, not other nearby cell types.
The scientists note that the direct killing of tumors by ROS they saw does not negate the possibility that tumor necrosis factor alpha also is working through its previously known method of killing off blood supplies to tumors.
Antioxidant therapy in patients with active cancer has drawn mixed results, but most studies indicate that it worsens cancer, particularly in smokers, according to the National Cancer Institute. Preclinical studies in mice indicate the therapy promotes tumor growth and metastasis. Studies exploring the benefit of antioxidant therapy in preventing cancer have largely shown no benefit or harm, the NCI says.
Tumors are known to impact T cells. In fact, scientists have shown that the two can compete for nutrition and energy in the tumor microenvironment, remote sites tumors establish to spread successfully, the scientists write. It's T cells that usually get short shrift in the struggle.
Comparatively, little focus has been on what T cells do to tumors, Zhou and his colleague's report. But a better understanding of that impact should help improve immunotherapies, like adoptive T cell therapy, which seek to enable T cells to better target tumors.
Adoptive T cell therapy is still under development for the treatment of colorectal cancer. This therapeutic approach was already known to essentially poke holes in cancer cells to kill them.
ROS are chemicals like peroxide and superoxide that are byproducts of necessary body functions like the use of oxygen and energy production by cell powerhouses called mitochondria. One reason cancer cells have naturally higher ROS levels is they have a high energy demand, Zhou says, constantly working to grow and spread.
Some level of ROS also benefits our healthy cells, including cell proliferation and differentiation. But, too much is also deadly to normal cells, even damaging to DNA.
Source-Eurekalert