Pfizer Inc announced an agreement with Schwarz Pharma AG under which it would be acquiring exclusive worldwide rights to fesoterodine
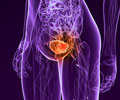
Pfizer Inc announced an agreement with Schwarz Pharma AG under which it would be acquiring exclusive worldwide rights to fesoterodine, a new drug that can be a treatment option for an overactive bladder.
"Overactive bladder is a debilitating condition that affects up to 100 million people around the world," said Karen Katen, vice chairman of Pfizer Inc and president, Pfizer Human Health. "We are excited about the potential of this new medicine to offer patients and their doctors a new alternative to existing therapies for managing the symptoms of overactive bladder." Schwarz had submitted an application for fesoterodine approval to both the US Food and Drug Administration and the European Medicines Evaluation Agency (EMEA) earlier this year. Initially Pfizer will pay $100 million to Schwarz Pharma. This amount can be increased provided the drug meets certain milestones. Royalties of both fesoterodine and Pfizer's Detrol product line for treatment of overactive bladder will be paid to Schwarz. Pfizer's Detrol LA is a leading drug that helps control involuntary bladder contractions and decreases the symptoms of urgency with or without frequency and incontinence. Since its introduction it has been prescribed to over 11 million patients worldwide.