One million packets of birth control pills have been recalled by Pfizer in the US over a packaging error that could put women at risk of unintended pregnancy.
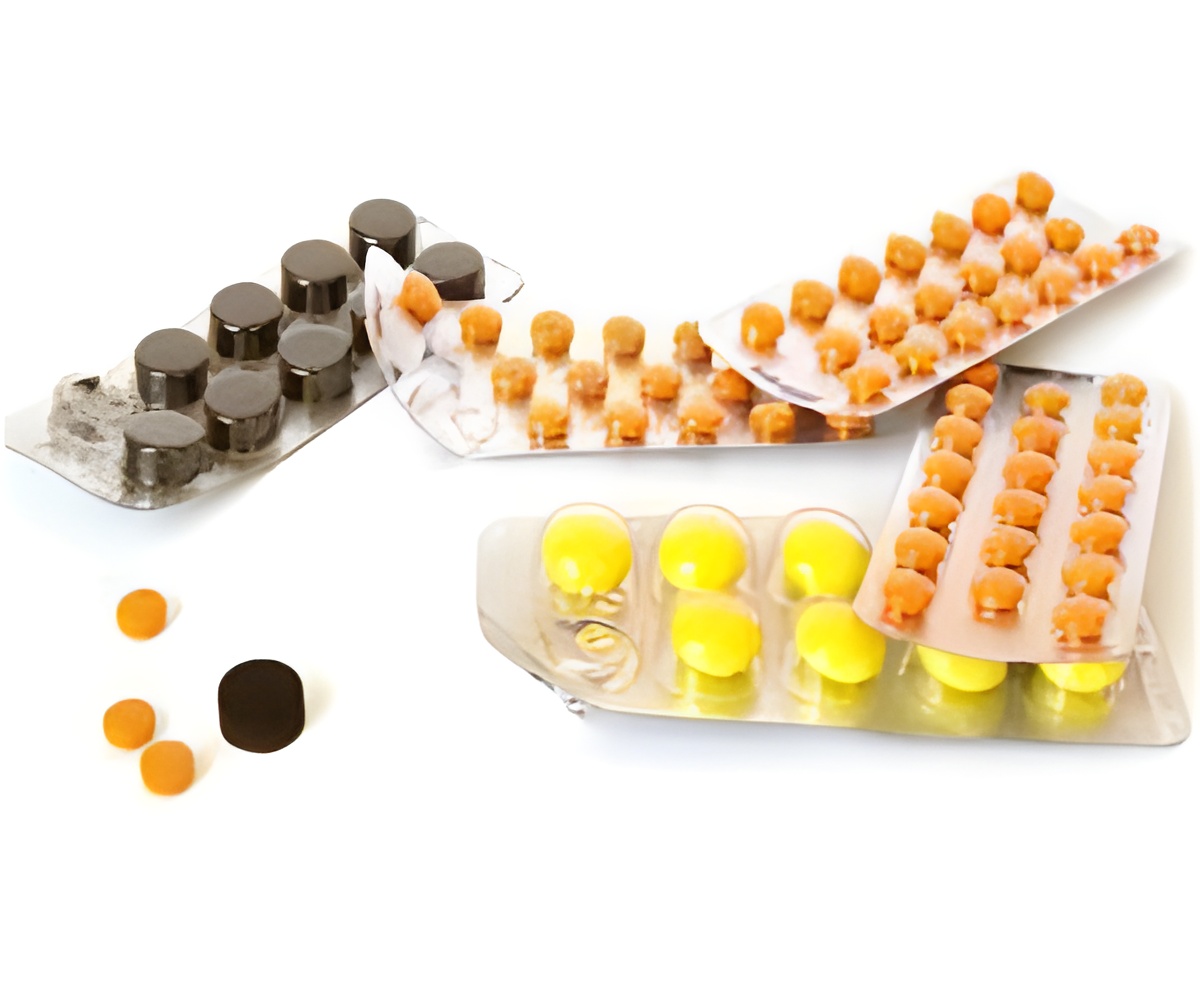
"An investigation by Pfizer found that some blister packs may contain an inexact count of inert or active ingredient tablets and that the tablets may be out of sequence," Pfizer said in a statement.
"As a result of this packaging error, the daily regimen for these oral contraceptives may be incorrect and could leave women without adequate contraception, and at risk for unintended pregnancy."
The pills were manufactured and packaged by Pfizer in New York state and commercialized by Akrimax Rx Products under the Akrimax brand name in the United States.
Each packet is supposed to contain 21 active tablets and seven inactive pills.
"The cause of the inexact package counts has been identified and corrected," Pfizer spokeswoman Grace Ann Arnold told AFP.
Advertisement
Patients who have been taking the affected lot numbers, posted on the US Food and Drug Administration website, should notify their physician, return the product to the pharmacy and begin using a back-up form of non-hormonal birth control right away, Pfizer said.
Advertisement