Churg-Strauss Syndrome is a rare autoimmune disease that causes inflammation and tissue damage. Mepolizumab is a drug that can reduce the need for steroid medications.
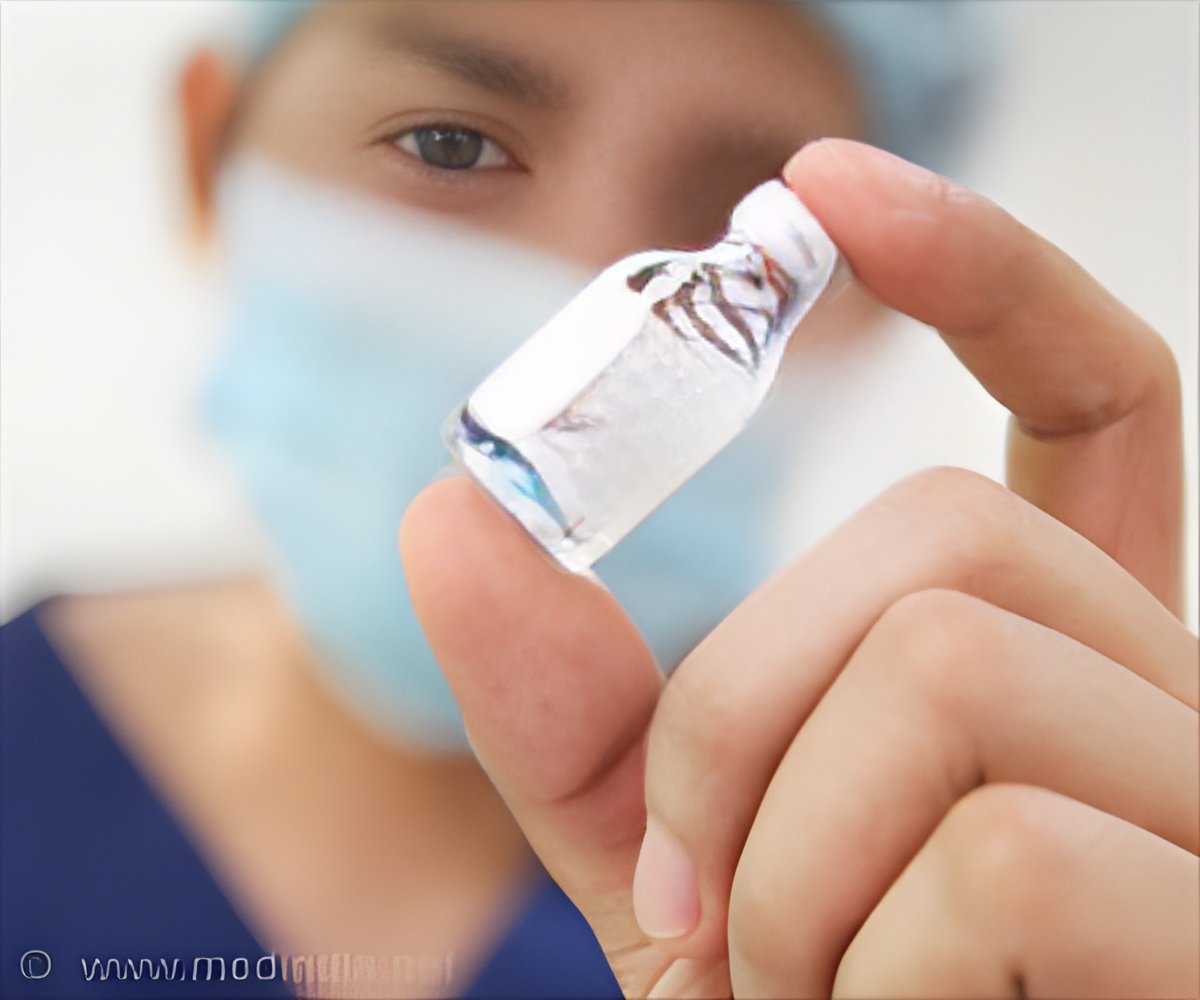
‘In phase III clinical trial, mepolizumab, a monoclonal antibody reduced the need for steroid medications in patients with Churg-Strauss Syndrome.’





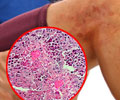
In Churg-Strauss Syndrome, also known as eosinophilic granulomatosis with polyangiitis, specific types of white blood cells known as eosinophils accumulate in tissue, causing inflammation and tissue damage. Patients usually develop severe asthma and rhinosinusitis and later suffer damage to the lungs and other organs. It is often misdiagnosed as asthma. There are 500 to 1,000 new cases of Churg-Strauss diagnosed each year in the United States.There is no cure and no approved treatment for the disease. It is most commonly treated with systemic corticosteroids and immunosuppressant medications, both of which come with significant side effects. In spite of these therapies, exacerbations of the disease, which may cause permanent organ damage, are common.
Mepolizumab is a monoclonal antibody that prevents the cytokine IL-5 from binding to receptors on the surface of eosinophils, which prevents their maturation, differentiation, and proliferation. It has been an effective medication for various eosinophilic disorders including severe eosinophilic asthma.
The researchers enrolled 136 patients with relapsing/refractory Churg-Strauss in the first double-blind, placebo-controlled trial of a medication for this disease. Patients continued with existing treatments of corticosteroids with or without additional immunosuppressant medications. Half received mepolizumab injections and half received placebo once every four weeks for 52 weeks.
For the primary outcome of remission, 28 percent of those receiving mepolizumab achieved remission for more than 24 weeks, compared to only 3 percent on placebo. Thirty-two percent of patients on mepolizumab were in remission at weeks 36 and 48, compared to only 3 percent of those receiving placebo. About half of patients receiving mepolizumab failed to achieve remission, compared to 80 percent of patients receiving placebo.
Advertisement
During the last four weeks of the study, 50 patients (44 percent) receiving mepolizumab were able to reduce their average steroid dose to less than 4 mg/day compared to only 5 patients (7 percent) on placebo. Twelve patients (18 percent) receiving mepolizumab were able to discontinue steroid treatment completely, compared to only 2 (3 percent) receiving placebo.
The findings are published in the New England Journal of Medicine.
Source-Eurekalert