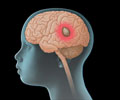
Promising advancements in brain tumor therapy are observed in human trials, where a pioneering gene therapy regimen exhibits both safety and efficacy.
‘Exciting progress emerges in the field of brain tumor treatment, as human trials reveal that a novel gene therapy combining cell-killing and immune-boosting drugs can safely and effectively prolong the survival of glioma patients.
# BrainTumor, #GeneTherapy
’





The therapy proved not only safe but also improved survival, reveals the study published in the journal The Lancet Oncology."Being able to move a novel therapy from bench to bedside in such a streamlined fashion is exciting, and represents a tour-de-force in translational medicine," said Oren Sagher, Professor of neurosurgery at the University of Michigan. In the phase 1 trial, the team focused on two types of genetic therapies in high-grade gliomas. The first was a combination of HSV-1-TK -- a protein -- and Valtrex -- a drug used to treat viral infections like cold sores and chickenpox.
HSV-1-TK turns Valtrex into a cytotoxic compound that kills actively dividing cancer cells. The second was Flt3L -- a protein that recruits essential immune cells to the brain. When used in combination, these therapies showed exciting early results, including improved survival.
Of the 18 patients enrolled in the trial, six survived more than two years, three survived more than three years, and one patient, who is still alive at the time of publication, survived up to five years.
Glioma Patients Rejoice
With the current standard-of-care, the life expectancy for a tumor of this kind is just over 14 months. Further, the study found that this treatment wasn't toxic to the patients, suggesting that the highest dose used in this trial could be used in future trials.Though the adenoviral gene therapy vectors were supposed to be active for up to a month, the team discovered that activity from the adenoviral vector expressing the HSV1-TK was active for up to 17 months.
Advertisement
"This originated from a theoretical idea based on evolutionary hypotheses and was first tested in experimental models of the disease," said Pedro Lowenstein, Professor of Neurosurgery at U-M.
Advertisement
Although more work is needed before this is brought to the clinic, the significance of long-term expression of HSV1-TK suggests implementation to improve treatment. The results of this study support the design of future phase 1b/2 clinical trials.
Source-IANS