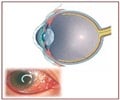
Administering the drug ranibizumab is associated with reducing the magnitude of legal blindness and visual impairment caused by age-related age-related macular degeneration, says study
To estimate the number of individuals in the United States who may benefit from treatment with ranibizumab to treat neovascular AMD (occurs when new blood vessels form in the retinal tissue, that can break easily, causing bleeding and damage to surrounding tissue) and prevent AMD-related blindness, Neil M. Bressler, M.D., of Wilmer Eye Institute, The Johns Hopkins University, Baltimore, and colleagues designed a modeling study using outcomes from three previous phase 3 ranibizumab trials.
Using statistics from the Beaver Dam Eye Study and data from the 2008 U.S. Census Bureau, the model predicted that 151,340 non-Hispanic white individuals in 2008 would develop neovascular AMD. Using data from the Age-Related Eye Disease Study (a phase 3 ranibizumab trial), the authors estimated that one-third of these cases (51,000 individuals) would already have preexisting choroidal neovascularization (new blood vessels form in the choroid, a thin vascular layer that supplies blood to the retina) in the opposite eye, making those patients ineligible for the model used in this study. Of the 151,340 individuals, the authors estimated that ranibizumab would be accessible to 103,582 individuals, making them eligible for inclusion in the study's modeling criteria.
Based on the model designed for the study, if no treatment were given to the 103,582 cases for which monthly ranibizumab was indicated and accessible, 16,268 (16 percent) would progress to legal blindness in two years. The authors estimated that monthly ranibizumab usage would reduce the incidence of legal blindness in two years by 72 percent, to 4,484 individuals. Additionally, based on the model designed for the study, if no treatment were applied to the 103,582 cases for which monthly ranibizumab is indicated and accessible, 34,702 (34 percent) would progress in two years to visual impairment (worse than 20/40 in the better-seeing eye, a level that precludes an unrestricted driver's license in most states). The authors estimated that monthly ranibizumab usage would reduce the incidence of visual impairment in two years by 37 percent, to 21,919 individuals.
Based on results of the model designed for this study, the authors conclude that ranibizumab would have an effect on reducing the occurrence of visual blindness in individuals with AMD when treatment is administered on a monthly basis when available.
Source-Eurekalert