Researchers demonstrate the safety and efficacy of using infusion pumps to administer Parkinson's medication.
Safety and efficacy of continuous subcutaneous levodopa-carbidopa infusion (ND0612) for Parkinson’s disease with motor fluctuations (BouNDless): a phase 3, randomised, double-blind, double-dummy, multicentre trial
Go to source). These results, published in the Lancet Neurology journal, could lead to additional treatment options for patients with the condition.
‘Did You Know?
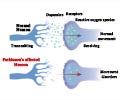
Dopamine-producing neurons in the brain gradually degenerate in Parkinson's disease, leading to characteristic motor symptoms such as tremors, stiffness, and slowness of movement. #parkinsondisease #medication #medindia’





Parkinson’s symptoms such as tremors, slowness, and stiffness are caused by low levels of dopamine in the body. For decades, doctors have treated Parkinson’s by giving patients levodopa, the inactive substance in the brain that once converted makes dopamine. Dopamine-producing neurons in the brain gradually degenerate in Parkinson's disease, leading to characteristic motor symptoms such as tremors, stiffness, and slowness of movement. #parkinsondisease #medication #medindia’
“Levodopa is a replacement strategy. We all make levodopa, but Parkinson's patients make less of it,” said Espay, co-principal investigator of the trial, James J. and Joan A. Gardner Family Center for Parkinson’s Disease Research Endowed Chair in UC’s Department of Neurology and Rehabilitation Medicine and a physician at the UC Gardner Neuroscience Institute.
Infusion Pump Therapy Redefines Symptom Management
Espay said oral levodopa is effective and typically helps people regain normal motor function, but its benefits tend to last less than a few hours after a few years, requiring increases in doses or frequency.Levodopa is most commonly administered orally, but this trial tested continuous, 24-hour levodopa delivery through a subcutaneous infusion pump.
A total of 381 patients with Parkinsons disease in 16 countries enrolled in the trial and were randomized to receive levodopa through the infusion pump or traditional oral medication.
The researchers found levodopa delivered through the infusion pump was safe and led to almost two hours of the day (1.72) of additional “on time,” or the time when the medication is working and symptoms are lessened, compared to taking levodopa orally.
Advertisement
“Once approved, this will become an important treatment strategy to consider for patients with Parkinson’s disease experiencing motor fluctuations not adequately controlled with medication,” he said.
Advertisement
Two additional subcutaneous delivery systems are also expected to be approved this year, Espay said, and researchers are continuing to study how to improve levodopa formulations and delivery to optimize its effect on patients.
“Levodopa delivery systems are expected to continue to improve over time,” he said. “This is a thriving area of research for the benefit of our patients.”
Reference:
- Safety and efficacy of continuous subcutaneous levodopa-carbidopa infusion (ND0612) for Parkinson’s disease with motor fluctuations (BouNDless): a phase 3, randomised, double-blind, double-dummy, multicentre trial - (https://www.sciencedirect.com/science/article/pii/S1474442224000528?via%3Dihub)
Source-Eurekalert