Topical JAK inhibitors are becoming a major significant force in atopic dermatitis space. Atopic dermatitis or eczema is an inflammatory, non-contagious, chronic skin disorder.
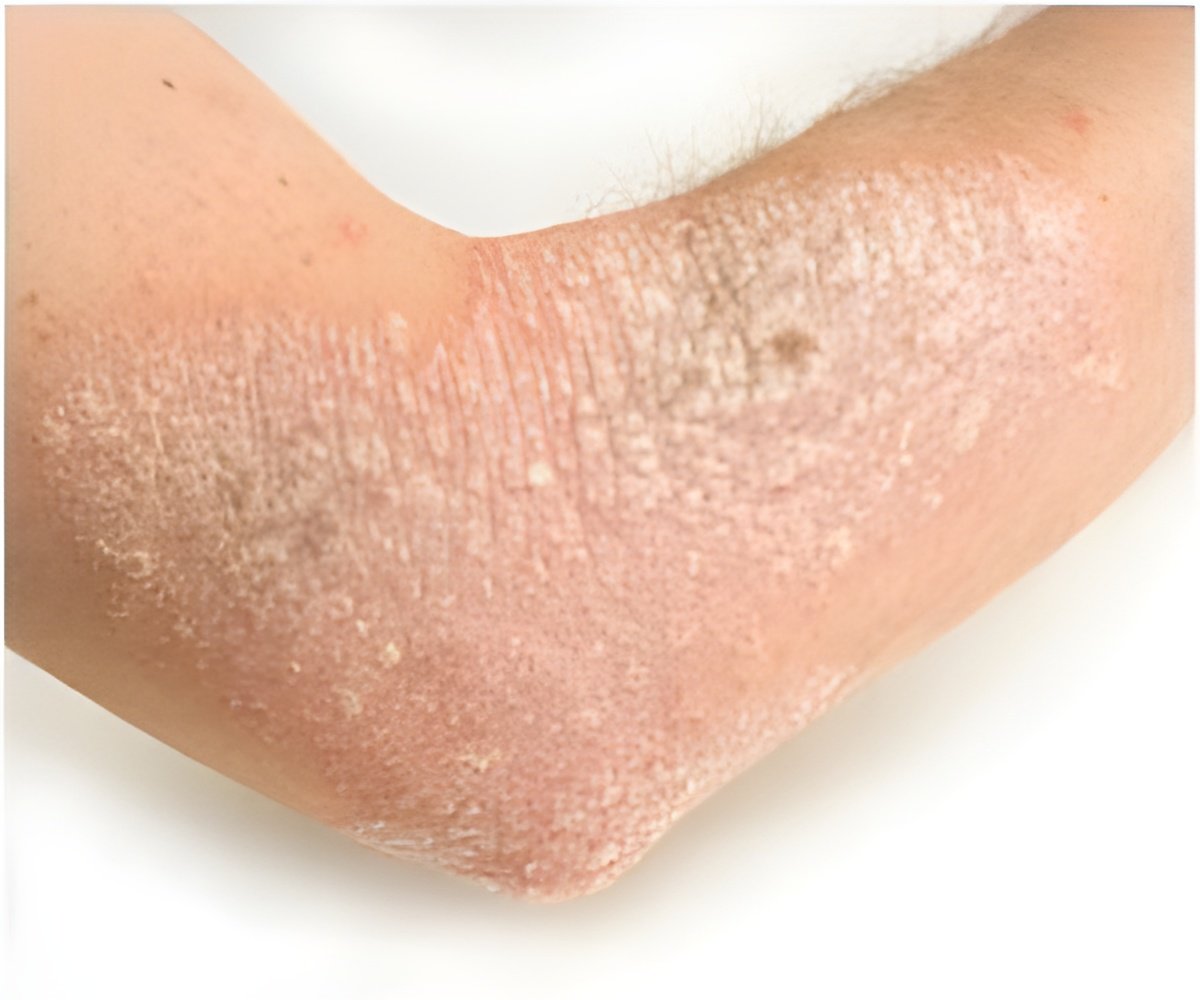
‘Topical JAK inhibitors drugs are progressing as main asset in the array of atopic dermatitis treatment.’





Thus, targeting an array of patient groups to tolerate the topical agents for mild-to-moderate cases can lead to better compliance. Though a relatively small amount of JAKs on the market now, they are set to revolutionize the AD space.Incyte’s ruxolitinib is a JAK1/2 inhibitor with strong efficacy that targets new patient pool before receiving approval is already marketed as Jakafi, an oral formulation for the treatment of myelofibrosis.
The company is anticipating approval in adults after successful clinical trials in children and is looking to greatly expand its market. Rival Pfizer used a similar tactic with its topical PDE4 inhibitor, Eucrisa (crisaborole).
LEO Pharma’s delgocitinib is a topical pan-JAK inhibitor used in chronic hand eczema. It is also Ruxolitinib’s closest competitor with fast Track Designation (FTD) that will help to expedite the regulatory process. It is marketed in Japan under the name Corectim for adults and more recently for pediatric patients with AD.
Aclaris Therapeutics’ ATI-1777 is an emollient spray administration that differentiates it from other competitors in CHE treatment.
Advertisement
Advertisement