Jevtana, the new chemotherapy treatment from Sanofi-Aventis, has now won approval in Europe soon after the US had granted it.
Successful clinical trials had shown that Jevtana (cabazitaxel) in conjunction with the steroid prednisone could be used in treating advanced second-line prostate cancer. The European Medicines Agency’s Committee for Medicinal Products for Human Use had expressed its approval after the phase III TROPIC clinical study, where this combination showed a significant survival benefit for patients with metastatic hormone-refractory prostate cancer (mHRPC). Hormone therapy is frequently the first treatment offered for mHRPC, with patients who no longer respond often receiving chemotherapy. But chemotherapy resistance could become the next hurdle, and the disease seemed to have no successful treatment strategy. But as Dr. Debasish Roychowdhury, head of Sanofi’s global oncology division states, “Jevtana in combination with prednisone/prednisolone reduced the risk of death by nearly one third and extended progression-free survival.”
Prostate cancer is the third most common cancer in men and the sixth biggest cancer killer in Europe. In this context, European approval of the drug would have a significant impact on patients in Europe.
Source-Medindia

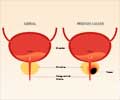
![Prostate Specific Antigen [PSA] & Prostate Cancer Diagnosis Prostate Specific Antigen [PSA] & Prostate Cancer Diagnosis](https://images.medindia.net/patientinfo/120_100/prostate-specific-antigen.jpg)