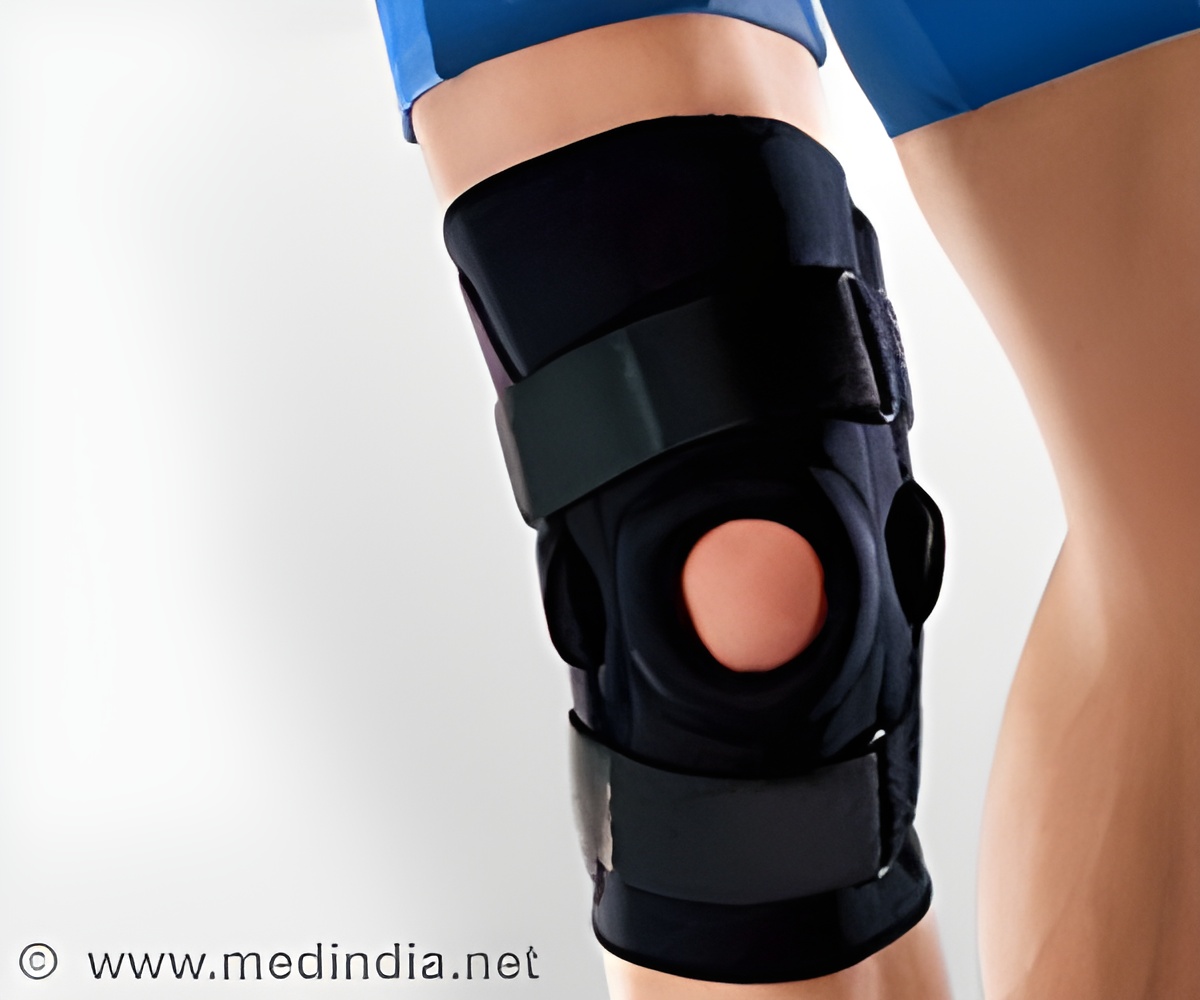
The muscle activation system consists of a brace with a built-in neuromuscular electrical stimulator that uses high intensity signals to activate the muscle.
Rob Morocco, President and CEO of CyMedica Orthopedics, said that securing the CE mark for the QB1 system was an important milestone for the company.
“The approval greatly expanded our market opportunity to patients in Europe and paving the way for a global sales and distribution network. We are excited to have the clearance to deliver our revolutionary technology to millions of patients outside of the United States," Morocco said.
QB1 system was designed to address quadriceps muscle atrophy that’s common after big knee surgeries.
According to the company, the muscle activation system consists of a brace with a built-in neuromuscular electrical stimulator that uses high intensity signals to activate the muscle. The high intensity signals are safe and will not produce the discomfort that would be expected from such an approach, the developers noted.
The system depends on closed-loop monitoring to regularly adapt the intensity of the signal to the comfort of the patient. A wired remote helps set and adjust various parameters of the QB1.
Advertisement
Source-Medindia